Abstract
What is the function of cognition? On one influential account, cognition evolved to co-ordinate behaviour with environmental change or complexity (Godfrey-Smith in Complexity and the function of mind in nature, Cambridge Studies in Philosophy and Biology, Cambridge University Press, Cambridge, 1996). Liberal interpretations of this view ascribe cognition to an extraordinarily broad set of biological systems—even bacteria, which modulate their activity in response to salient external cues, would seem to qualify as cognitive agents. However, equating cognition with adaptive flexibility per se glosses over important distinctions in the way biological organisms deal with environmental complexity. Drawing on contemporary advances in theoretical biology and computational neuroscience, we cash these distinctions out in terms of different kinds of generative models, and the representational and uncertainty-resolving capacities they afford. This analysis leads us to propose a formal criterion for delineating cognition from other, more pervasive forms of adaptive plasticity. On this view, biological cognition is rooted in a particular kind of functional organisation; namely, that which enables the agent to detach from the present and engage in counterfactual (active) inference.


Similar content being viewed by others
Notes
For broader philosophical discussion of these ideas in the context of predictive processing, see Clark (2016), Hohwy (2013) and Wiese and Metzinger (2017). For more technical explications of the free energy principle and its corollaries, see Bogacz (2017), Buckley et al. (2017) and Friston et al. (2017a).
Variational inference techniques are also widely used in machine learning to approximate density functions through optimisation (see Blei et al. 2017).
Of course, just because a system can be described as behaving in a way that minimises variational free energy (maximises Bayesian model evidence, approximates Bayesian inference, etc.) does not guarantee that it actually implements any such computation. The extent to which the free energy principle should be construed as a useful heuristic for describing and predicting adaptive behaviour (a kind of intentional stance; Dennett 1987), versus a more substantive ontological claim, remains an open question. That said, recent progress has been made towards casting the free energy principle as a process theory of considerable explanatory ambition (Friston et al. 2017a).
Note that we interpret the notion of regulation rather broadly here. For philosophical arguments distinguishing regulation from related concepts such as feedback control and homeostasis, see Bich et al. (2016). On this view, regulatory control consists in a special kind of functional organisation characterised in terms of second-order control. This formulation seems broadly in line with our understanding of allostasis (see “Beyond homeostasis: Allostasis and hierarchical generative models”).
Note that the organism’s morphology and internal organisation impose constraints on the way it models and represents environmental dynamics (e.g., Parr and Friston 2018a)—a point we shall elaborate in “Biological regulation in an uncertain world”.
See Parr and Friston (2018b) for a mathematical explanation of the (bound) relationship between variational free energy and model evidence.
While active inference is sometimes narrowly construed as the active or behavioural component of the perception–action loop, the term was originally introduced to characterise the reciprocal interplay between perception and action (e.g., Friston et al. 2009, p. 4). This broader interpretation emphasises the deep continuity of the (Bayesian inferential) processes underwriting perception, learning, planning, and action under the free energy principle (Friston et al. 2017a).
This general understanding of perception need not entail the conscious experience of sensations, just as learning can occur through entirely unconscious—and even artificial—mechanisms. Rather, what is at stake here is the statistical notion of Bayesian belief, where probability distributions encode the conditional probability that sensory observation Y was caused by hidden state X.
Technically, actions are physical, real-world states that are not represented within the agent’s generative model (Attias 2003). Rather, the agent infers (fictive) ‘control’ states that explain the (sensory) consequences of its actions (Friston et al. 2012a, d). Action selection (or decision-making) thus amounts to the optimisation of posterior beliefs about the control states that determine hidden state transitions (Friston et al. 2013, 2015b).
Although one might be tempted to subordinate perceptual inference to free energy minimising action, we interpret perception and action as mutually dependent moments within a unified dynamical loop (cf. the perception–action cycle; Fuster 2001, 2004). Ultimately, both modes of active inference are in the service of uncertainty reduction: Percepts without actions are idle; actions without percepts are blind.
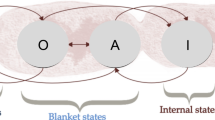
Formally speaking, the sensory and active states that compose the Markov blanket render the probability distributions over internal and external states statistically independent of one another (see Pearl 1988). In other words, internal and external states provide no additional information about one another once the Markov blanket’s active and sensory states are known.
Although we focus here on allostasis, numerous other concepts emphasising the dynamic nature of biological regulation have been proposed in an effort to extend (or transcend) classical notions of homeostatic setpoint control (see for e.g., Bauman 2000; Berntson and Cacioppo 2000 and references therein).
This formulation is congruent with contemporary efforts to finesse traditional notions of setpoint rigidity with more dynamic accounts of homeostatic control (e.g., Cabanac 2006; Ramsay and Woods 2014; cf. Ashby 1940). It also seems more felicitous to Cannon’s original conception of homeostatic control (see for e.g., Cannon 1939, p. 39).
Note that priors over certain physiological variables (e.g., core temperature, blood pH) are likely to be held with greater precision—and thus restricted to a narrower range of attracting states—than others (e.g., blood pressure, heart rate; see Allen and Tsakiris 2018; Seth and Friston 2016; Yon et al. 2019).
One might protest that all we have done here is pivot from one sort of reactive homeostatic mechanism to another; albeit, one involving responses to an external (rather than internal) threat. Nevertheless, we consider this simple scenario as exemplary of the fundamental principle of allostatic regulation; namely, the modulation of physiological states in anticipation of future conditions, and in the absence of any immediate homeostatic perturbation. This example can easily be extended to capture a rich assortment of allostatic dynamics that play out across increasing levels of abstraction and spatiotemporal scale.
Note that the appeal to expected free energy was also implicit in the predator example of the previous section, insofar as transient increases in homeostatic prediction error were tolerated in order to avoid a much more surprising fate—being eaten!
See Moore (2004) for a thoroughgoing review of such associative learning mechanisms.
More precisely, this capacity depends on the ability to infer the expected free energy of the outcomes associated with various potential state trajectories, as well as the expected likelihood of outcomes under each policy (see Friston et al. 2017a, c; Parr and Friston 2017, 2018b). We have suggested such inferential processes might be facilitated by the co-ordination of exteroceptive sampling and motor activity with periodic regimes of autonomic/interoceptive active inference (Corcoran et al. 2018).
We emphasise again that the conscious, reflective character of these intuitive examples should not detract from the idea that the possibility of such experiences is underwritten by more basic, unconscious allostatic mechanisms. For example, the growth onset of a horse’s winter coat is not assumed to represent a strategic decision on the part of the horse, but rather a physiological response to seasonal changes in photoperiod. Similarly, a rabbit might schedule her foraging bouts to balance energy gain against predation risk, even though she might not be capable of representing and evaluating these concerns explicitly (this trade-off may, for instance, be implicitly encoded within the animal’s circadian rhythm—see “Model 2: Hierarchical active inference”).
This gloss on the environmental complexity thesis is reminiscent of W. Ross Ashby’s law of requisite variety (1956, 1958; cf. Conant and Ashby 1970), and is clearly in line with recent neuroscientific interest in the brain’s teleonomic function as a sophisticated biological regulator (for discussion, see Williams and Colling 2018). Although Godfrey-Smith (1996, pp. 76–79) briefly remarks upon the connection between cybernetic accounts of homeostatic control and cognitive function, he rejects their strong continuity on the grounds that cognition can sustain biological viability through actions that circumvent homeostatic mechanisms. We concur that non-trivial definitions of homeostasis and cognition invoke concepts that are distinct from one another, and argue below that this distinction can be cashed out in terms of their constitutive inferential architectures.
In fact, real E. coli realise a similar ‘adaptive gradient climbing’ strategy by integrating chemosensory information about the ambient chemical environment over time, and modulating the probability of tumbling as a function of attractant rate of change (Berg and Brown 1972; Falke et al. 1997). More recent work has indicated that such chemotactic activity approximates optimal Kalman filtering (Andrews et al. 2006), where hidden states are estimated on the basis of prior and present observations weighted by their uncertainty (Kalman 1960; Kalman and Bucy 1961; see Grush 2004, for discussion). As Kalman filtering constitutes a special case of Bayesian filtering (one that is equivalent to predictive coding; Bastos et al. 2012; Friston et al. 2010b, 2018), chemotaxis can be cast as a gradient descent on variational free energy. Notice that our model is deliberately simpler than this scheme, since sensory prediction errors are not modulated by an uncertainty (precision) parameter.
The story changes if the organism’s receptors are compatible with molecules it cannot metabolise, or that afford low nutritional value (assuming such molecules are prevalent enough to significantly interfere with chemotaxis). See Sterelny (2003, pp. 20–26) for discussion of the challenges posed by ‘informationally translucent environments’ that confront organisms with ambiguous (or misleading) cues. Environmental translucence calls for greater model complexity; e.g., the capacity to integrate information harvested across multiple sensory channels (cf. robust tracking; Sterelny 2003, pp. 27–29).
Indeed, one might construe the minimal model as a simplified analogue of Ashby’s (1960) ‘Homeostat’.
See Godfrey-Smith (2016b) for a complementary discussion of this topic in relation to microbial proto-cognition and metabolic regulation.
One might call this entity a Spencerian creature; i.e. an organism that responds to environmental change through “the continuous adjustment of internal relations to external relations” (Spencer 1867, p. 82; see discussion in Godfrey-Smith 1996, pp. 70–71). From an active inference perspective, this creature is the embodiment of pure perception; i.e. an organism that reconfigures its internal states (updates its model) in accordance with external conditions, without ever seeking to alter such conditions (cf. Bruineberg et al. 2018; Corcoran 2019).
One might play with the idea of entities that could exist like this quite happily once the ideal, invariant niche is discovered—perhaps deep within rocky crevices or underwater (one is reminded of the sea squirt that consumes its own brain after settling upon a permanent home, but the anecdote turns out to be an exaggeration; see Mackie and Burighel 2005). However, entities of this sort would surely fail to qualify as adaptive biological systems—at least insofar as the notion of adaptability implies some capacity to maintain one’s viability in the face of time-varying environmental dynamics (cf. ‘mere’ vs. ‘adaptive’ active inference; Kirchhoff et al. 2018). Moreover, such entities would also fail to qualify as agents in any biologically relevant sense (see for e.g., Moreno and Etxeberria 2005).
Interestingly, this scenario is reminiscent of a common criticism levelled against the free energy principle: the so-called dark-room problem (Friston et al. 2012e). The thrust of this argument is that free energy minimisation should compel agents to seek out the least-surprising environments possible (e.g., a room devoid of stimulation) and stay there until perishing. Various rejoinders to this charge have been made (see for e.g., Clark 2018; Hohwy 2013; Schwartenbeck et al. 2013), including the observation that this strategy will inevitably lead to increasing free energy on account of accumulating interoceptive prediction error (Corcoran 2019; Pezzulo et al. 2015). More technically, “itinerant dynamics in the environment preclude simple solutions to avoiding surprise” (Friston et al. 2009, p. 2), where the environment referred to here includes the biophysical conditions that obtain within the organism, as well as without. This is to say that the attractors around which adaptive biological systems self-organise are inherently unstable—both autopoietic (‘self-creating’) and autovitiating (‘self-destroying’)—thus inducing itinerant trajectories (heteroclinic cycles) through state-space (Friston 2011, 2012b; Friston and Ao 2012; Friston et al. 2012c).
In other words, dark rooms may very well appeal to creatures like us (e.g., as homeostatic sleep pressure peaks towards the end of the day), but the value such environments afford will inevitably decay as alternative possibilities (e.g., leaving the room to find breakfast after a good night’s sleep) become more salient and attractive (cf. alliesthesia, the modulation of affective and motivational states according to (time-evolving) physiological conditions; Berridge 2004; Cabanac 1971).
Note that the allostatic treatment of circadian regulation may in principle be extended to periodic phenomena spanning shorter or longer timescales; e.g., ultradian and circannual rhythms.
This scenario is not meant to imply that circadian rhythms are actually acquired in this fashion (although they are clearly susceptible to modulation through external cues). Rather, the idea we are trying to illustrate here is the way hierarchical architectures ground adaptive regulation over longer timescales by dint of their capacity to capture recurrent, slowly evolving patterns of environmental variation.
Notice that the agent forms a representation of a hidden cause corresponding to diurnal patterns of temperature variation despite its lack of exteroceptive sensitivity to such variables as temperature, viscosity, light, etc. Rather, it detects regular changes in its dynamics that cannot be ascribed to its own actions (which average out across the 24 h period), and infers some hidden external process as being responsible for these changes. It might not be right to say the agent represents ambient temperature per se, nor indeed the higher-order causes of the latter’s oscillation (sun exposure, planetary rotation, etc.). Our agent lacks sufficient hierarchical depth to arrive at such conclusions, collapsing these fine-grained distinctions into a fairly ‘flat’, undifferentiated representation of diurnal variation.
The remarkable robustness of circadian oscillations is thrown into relief whenever one traverses several time-zones—a good example of how strongly-held (i.e. high-precision or ‘stubborn’; see Yon et al. 2019) allostatic expectations persist in the face of contradictory sensory evidence (i.e. the phase-shifted photoperiod and feeding schedule, to which the system eventually recalibrates; Asher and Sassone-Corsi 2015; Menaker et al. 2013).
Note that our use of counterfactual semantics here is not intended to imply that cognition bears any necessary resemblance to linguistic processing; it is simply adopted as a convenient way of characterising the logic of model selection under active inference.
Interestingly, recent psychological evidence suggests that counterfactual scenarios deemed more similar to previously experienced events are perceived as more plausible and easier to envisage (i.e. simulate) than more distant alternatives (Stanley et al. 2017). This observation lends weight to the idea that humans evaluate competing counterfactual predictions in accordance with their proximity to actual states of affairs, where proximity or similarity might be cashed out in terms of (Bayesian) model evidence (see Fitzgerald et al. 2014).
Risk and ambiguity are also known as irreducible uncertainty and (parameter) estimation uncertainty, respectively (de Berker et al. 2016; Payzan-LeNestour and Bossaerts 2011). Note that uncertainty can be decomposed in various other ways, depending on the domain of interest (see for e.g., Bland and Schaefer 2012; Bradley and Drechsler 2014; Kozyreva and Hertwig 2019).
This characterisation of risk and ambiguity is broadly consistent with descriptions in economics (e.g., Camerer and Weber 1992; Ellsberg 1961; Kahneman and Tversky 1979; Knight 1921) and neuroscience (e.g., Daw et al. 2005; Hsu et al. 2005; Huettel et al. 2006; Levy et al. 2010; Payzan-LeNestour and Bossaerts 2011; Preuschoff et al. 2008; for a review, see Bach and Dolan 2012). Importantly, these two sorts of uncertainty rest upon the precision (inverse variability) of the likelihood mapping between outcomes and hidden states—and transitions amongst hidden states that may or may not be under the creature’s control. Technically, the first sort of precision relates to observation noise, while the second relates to system or state noise, i.e. volatility. Formally, volatility can be construed as the (inverse) precision over transition probabilities (i.e. confidence about the way hidden states evolve over time; Parr and Friston 2017; Parr et al. 2019; Sales et al. 2019; Vincent et al. 2019). This formulation suggests that volatile environments will tend to generate more surprising outcomes than stable environments, insofar as their states are apt to change in ways that are difficult to anticipate. Note that the term volatility is used differently in various contexts (see for e.g., Behrens et al. 2007; Bland and Schaefer 2012; Mathys et al. 2014).
One caveat to this claim is that the (neuro)physiological mechanisms and cognitive operations required to enrich and exploit counterfactual predictive models may themselves engender additional costs (e.g., planning a new course of action requires time, energy, and effort; see Zénon et al. 2018). We assume that the costs incurred by such processes ‘pay for themselves’ over the long-run (or at least tend to on average), insofar as they enable the agent to exploit prior experience in ways that are conducive to adaptive behaviour (see Buzsáki et al. 2014; Pezzulo 2014; Pezzulo et al. 2017; Suddendorf et al. 2018). It is also worth pointing out that some of the costs engendered by counterfactual inference-supporting architectures may be mitigated by a variety of adaptive strategies (e.g., model updating during sleep, habitisation of behaviour under stable and predictable conditions; see Fitzgerald et al. 2014; Friston et al. 2017b; Hobson and Friston 2012; Pezzulo et al. 2016).
For the purposes of this brief discussion, we limit the scope of epistemic action to instances where the organism actively intervenes on its environment in order to resolve uncertainty. It is worth noting, however, that the concept can also refer to mental actions or cognitive operations that reduce uncertainty (see for e.g., Metzinger 2017; Pezzulo et al. 2016; Pezzulo 2017). On this broader understanding, one might construe the different varieties of counterfactual processing described above as covert modes of epistemic action.
Such activity is sometimes referred to as epistemic foraging, where the agent seeks out information about the way state transitions are likely to unfold (Friston et al. 2017d; Mirza et al. 2016; Parr and Friston 2017). For a nice example of epistemic foraging in wild dolphins, see Arranz et al. (2018).
It is interesting to remark how epistmic action contributes to the practical utility of cognition as understood under the environmental complexity thesis. Following Dewey (1929), Godfrey-Smith (1996, pp. 116–120) notes that cognition is most likely to be useful in environments that comprise a mixture of regularity and unpredictability. Specifically, distal states should vary in ways that are a priori unpredictable (but worth knowing about), while maintaining a stable relationship with proximal states (see also Dunlap and Stephens 2016). The capacity to engage in epistemic action enhances the potential utility of cognition precisely insofar as it helps the agent to reduce uncertainty over this mapping, thus affording more precise knowledge (or novel insight; Friston et al. 2017b) about the state of the world and its possible alternatives.
Godfrey-Smith thus rejects strong continuity, the view that “[l]ife and mind have a common abstract pattern or set of basic organizational properties. […] Mind is literally life-like” (1995, p. 320, emphasis in original). Evan Thompson (2007) has defended a position similar to this (‘deep continuity’), albeit with the addition of an existential-phenomenological supplement (for discussion, see Wheeler 2011). This view inherits from Maturana’s canonical account of autopoiesis, where one finds the strongest expression of life–mind continuity: “Living systems are cognitive systems, and living as a process is a process of cognition” (Maturana and Varela 1980, p. 13, emphasis added; see also Heschl 1990).
It is perhaps worth noting that other scholars have used the criterion of “detachment” (or “decouplability”) to distinguish representational versus non-representational agents, rather than cognitive versus non-cognitive agents (cf. Clark and Grush 1999; Grush 2004). Without digressing into a discussion of the relationship between representational and cognitive systems, we remark that our view conceives of cognition as a computational architecture that engages in a particular subset of representational operations—i.e. the generation, manipulation, and evaluation of counterfactual model predictions. These operations are situated within a broader class of uncertainty-resolving processes, including the homeostatic and allostatic representational schemes outlined in “Biological regulation in an uncertain world”.
‘Minimal cognition’ is perhaps more closely associated with a rather different set of philosophical views than those espoused by Godfrey-Smith (e.g., anti-representationalism, situated and embodied cognition; Barandiaran and Moreno 2006; Beer 2003; van Duijn et al. 2006). We take the main thrust of our argument to be equally applicable to these positions.
When pressed, Godfrey-Smith seems to hold this view: “I do not claim that bacteria exhibit cognition; this is at most a case of proto-cognition” (2002, p. 223, emphasis added).
References
Abe H, Lee D (2011) Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron 70(4):731–741
Adams F (2018) Cognition wars. Stud Hist Philos Sci 68:20–30
Adams RA, Shipp S, Friston KJ (2013) Predictions not commands: active inference in the motor system. Brain Struct Funct 218(3):611–643
Ainley V, Apps MAJ, Fotopoulou A, Tsakiris M (2016) ‘Bodily precision’: a predictive coding account of individual differences in interoceptive accuracy. Philos Trans R Soc B 371(20160003):1–9
Allen M, Friston KJ (2018) From cognitivism to autopoiesis: towards a computational framework for the embodied mind. Synthese 195(6):2459–2482
Allen M, Tsakiris M (2018) The body as first prior: Interoceptive predictive processing and the primacy of self-models. In: Tsakiris M, De Preester H (eds) The interoceptive mind: from homeostasis to awareness. Oxford University Press, Oxford, pp 27–45
Allen M, Levy A, Parr T, Friston KJ (2019) In the body’s eye: the computational anatomy of interoceptive inference. bioRxiv
Andrews BW, Yi T-M, Iglesias PA (2006) Optimal noise filtering in the chemotactic response of Escherichia coli. PLoS Comput Biol 2(11):e154
Arranz P, Benoit-Bird KJ, Southall BL, Calambokidis J, Friedlaender AS, Tyack PL (2018) Risso’s dolphins plan foraging dives. J Exp Biol 221(4):jeb165209
Ashby WR (1940) Adaptiveness and equilibrium. Br J Psychiatry 86(362):478–483
Ashby WR (1956) An introduction to cybernetics. Chapman & Hall Ltd, London
Ashby WR (1958) Requisite variety and its implications for the control of complex systems. Cybernetica 1(2):83–99
Ashby WR (1960) Design for a brain: The origin of adaptive behaviour, 2nd edn. Chapman & Hall Ltd., London
Asher G, Sassone-Corsi P (2015) Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161(1):84–92
Attias H (2003) Planning by probabilistic inference. In: Bishop CM, Frey BJ (eds) Proceedings of the ninth international conference on artificial intelligence and statistics. Society for Artificial Intelligence and Statistics, New Jersey
Bach DR, Dolan RJ (2012) Knowing how much you don’t know: a neural organization of uncertainty estimates neural organization of uncertainty estimates. Nat Rev Neurosci 13:572–586
Badcock PB, Davey CG, Whittle S, Allen NB, Friston KJ (2017) The depressed brain: an evolutionary systems theory. Trends Cognitive Sci 21(3):182–194
Badre D (2008) Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cognitive Sci 12(5):193–200
Bailey SM, Udoh US, Young ME (2014) Circadian regulation of metabolism. J Endocrinol 222(2):R75–R96
Baltieri M, Buckley CL (2017) An active inference implementation of phototaxis. In: Knibbe C, Beslon G, Parsons D, Misevic JR-C, Bredèche N, Hassas S, Simonin O, Soula H (eds) Proceedings of ECAL 2017: the 14th European conference on artificial life. MIT Press, Cambridge, pp 36–43
Baluška F, Levin M (2016) On having no head: cognition throughout biological systems. Front Psychol 7:902
Barandiaran XE, Moreno A (2006) On what makes certain dynamical systems cognitive: a minimally cognitive organization program. Adapt Behav 14(2):171–185
Barrett LF, Simmons WK (2015) Interoceptive predictions in the brain. Nat Rev Neurosci 16(7):419–429
Barrett LF, Quigley KS, Hamilton P (2016) An active inference theory of allostasis and interoception in depression. Philos Trans R Soc B 371(20160011):1–17
Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ (2012) Canonical microcircuits for predictive coding. Neuron 76(4):695–711
Bauman DE (2000) Regulation of nutrient partitioning during lactation: Homeostasis and homeorhesis revisited. In: Cronjé PB (ed) Ruminant physiology: digestion, metabolism, growth and reproduction, chapter 18. CABI Publishing, New York, pp 311–328
Bechtel W (2011) Representing time of day in circadian clocks. In: Newen A, Bartels A, Jung E-M (eds) Knowledge and representation, Chapter 7. CSLI Publications, Stanford, pp 129–162
Beer RD (2003) The dynamics of active categorical perception in an evolved model agent. Adapt Behav 11(4):209–243
Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS (2007) Learning the value of information in an uncertain world. Nat Neurosci 10(9):1214–1221
Ben-Jacob E (2009) Learning from bacteria about natural information processing. Ann N Y Acad Sci 1178:78–90
Berg HC, Brown DA (1972) Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature 239(5374):500–504
Bernard C (1974) Lectures on the phenomena of life common to animals and plants. American Lecture Series. Charles C. Thomas Pub Ltd, Springfield
Berntson GG, Cacioppo JT (2000) From homeostasis to allodynamic regulation. In: Cacioppo JT, Tassinary LG, Berntson GG (eds) Handbook of psychophysiology, Chapter 17, 2nd edn. Cambridge University Press, Cambridge, pp 459–481
Berridge KC (2004) Motivation concepts in behavioral neuroscience. Physiol Behav 81(2):179–209
Bich L, Mossio M, Ruiz-Mirazo K, Moreno A (2016) Biological regulation: controlling the system from within. Biol Philos 31(2):237–265
Birkhoff GD (1931) Proof of the ergodic theorem. Proc Natl Acad Sci 17(12):656–660
Bland AR, Schaefer A (2012) Different varieties of uncertainty in human decision-making. Front Neurosci 6:85
Blei DM, Kucukelbir A, McAuliffe JD (2017) Variational inference: a review for statisticians. J Am Stat Assoc 112(518):859–877
Bogacz R (2017) A tutorial on the free-energy framework for modelling perception and learning. J Math Psychol 76:198–211
Botvinick M, Toussaint M (2012) Planning as inference. Trends Cogn Sci 16(10):485–488
Bradley R, Drechsler M (2014) Types of uncertainty. Erkenntnis 79(6):1225–1248
Brown H, Adams RA, Parees I, Edwards M, Friston KJ (2013) Active inference, sensory attenuation and illusions. Cogn Process 14(4):411–427
Bruineberg J, Rietveld E, Parr T, van Maanen L, Friston KJ (2018) Free-energy minimization in joint agent-environment systems: a niche construction perspective. J Theor Biol 455:161–178
Buckley CL, Chang SK, McGregor S, Seth AK (2017) The free energy principle for action and perception: a mathematical review. J Math Psychol 81:55–79
Buckner RL, Carroll DC (2007) Self-projection and the brain. Trends Cogn Sci 11(2):49–57
Bugnyar T, Reber SA, Buckner C (2016) Ravens attribute visual access to unseen competitors. Nat Commun 7:10506
Burdakov D (2019) Reactive and predictive homeostasis: roles of orexin/hypocretin neurons. Neuropharmacology 154:61–67
Buzsáki G, Peyrache A, Kubie J (2014) Emergence of cognition from action. Cold Spring Harb Symp Quant Biol 79:41–50
Cabanac M (1971) Physiological role of pleasure. Science 173(4002):1103–1107
Cabanac M (2006) Adjustable set point: to honor Harold T. Hammel. J Appl Physiol 100(4):1338–1346
Calvo P, Friston KJ (2017) Predicting green: really radical (plant) predictive processing. J R Soc Interface 14(20170096):1–11
Calvo Garzón P, Keijzer F (2011) Plants: adaptive behavior, root-brains, and minimal cognition. Adapt Behav 19(3):155–171
Camerer C, Weber M (1992) Recent developments in modeling preferences: uncertainty and ambiguity. J Risk Uncertain 5:325–370
Campbell JO (2016) Universal Darwinism as a process of Bayesian inference. Front Syst Neurosci 10:49
Cannon WB (1914) The emergency function of the adrenal medulla in pain and the major emotions. Am J Physiol 33(2):356–372
Cannon WB (1915) Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. D. Appleton and Company, New York
Cannon WB (1929) Organization for physiological homeostasis. Physiol Rev 9(3):399–431
Cannon WB (1939) The wisdom of the body: revised and, enlarged edn. W. W. Norton & Company Inc., New York
Carruthers P (2004) On being simple minded. Am Philos Q 41(3):205–220
Clark A (2015) Radical predictive processing. South J Philos 53:3–27
Clark A (2016) Surfing uncertainty: prediction, action, and the embodied mind. Oxford University Press, Oxford
Clark A (2017) How to knit your own markov blanket: resisting the second law with metamorphic minds. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 3. MIND Group, Frankfurt am Main, pp 1–19
Clark A (2018) A nice surprise? Predictive processing and the active pursuit of novelty. Phenomenol Cogn Sci 17(3):521–534
Clark A, Grush R (1999) Toward a cognitive robotics. Adapt Behav 7(1):5–16
Conant RC, Ashby WR (1970) Every good regulator of a system must be a model of that system. Int J Syst Sci 1(2):89–97
Corcoran AW (2019) Cephalopod molluscs, causal models, and curious minds. Anim Sentience 4(26):13
Corcoran AW, Hohwy J (2018) Allostasis, interoception, and the free energy principle: feeling our way forward. In: Tsakiris M, De Preester H (eds) The interoceptive mind: from homeostasis to awareness, Chapter 15. Oxford University Press, Oxford, pp 272–292
Corcoran AW, Pezzulo G, Hohwy J (2018) Commentary: Respiration-entrained brain rhythms are global but often overlooked. Front Syst Neurosci 12:25
Corcoran AW, Pezzulo G, Hohwy J (2019) From allostatic agents to counterfactual cognisers: active inference, biological regulation, and the origins of cognition. Preprints, 2019110083
Craig AD (2009) How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10(1):59–70
Craik K (1943) The nature of explanation. Cambridge University Press, Cambridge
Crauel H, Flandoli F (1994) Attractors for random dynamical systems. Probab Theory Relat Fields 100:365–393
Critchley HD, Harrison NA (2013) Visceral influences on brain and behavior. Neuron 77(4):624–638
Dampney RAL (2016) Central neural control of the cardiovascular system: current perspectives. Adv Physiol Educ 40(3):283–296
Daw ND, Niv Y, Dayan P (2005) Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8(12):1704–1711
de Berker AO, Rutledge RB, Mathys C, Marshall L, Cross GF, Dolan RJ, Bestmann S (2016) Computations of uncertainty mediate acute stress responses in humans. Nat Commun 7:10996
de Vries B, Friston KJ (2017) A factor graph description of deep temporal active inference. Front Comput Neurosci 11:95
Degaute JP, van de Borne P, Linkowski P, Van Cauter E (1991) Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 18(2):199–210
Dennett DC (1987) The intentional stance. MIT Press, Cambridge
Dennett DC (1995) Darwin’s dangerous idea: evolution and the meanings of life. Penguin Books Ltd, London
Dewey J (1929) Experience and nature. George Allen & Unwin Ltd, London
Dolan RJ, Dayan P (2013) Goals and habits in the brain. Neuron 80(2):312–325
Dunlap AS, Stephens DW (2016) Reliability, uncertainty, and costs in the evolution of animal learning. Curr Opin Behav Sci 12:73–79
Dworkin BR (1993) Learning and physiological regulation. University of Chicago Press, Chicago
Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco-Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschöp MH, Eckel-Mahan K, Sassone-Corsi P (2018) Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 174(6):1571–1585
Elias P (1955) Predictive coding—part I. IRE Trans Inf Theory 1(1):16–24
Ellsberg D (1961) Risk, ambiguity, and the Savage axioms. Q J Econ 75(4):643–669
Evans DJ, Searles DJ (1994) Equilibrium microstates which generate second law violating steady states. Phys Rev E 50(2):1645–1648
Evans DJ, Searles DJ (2002) The fluctuation theorem. Adv Phys 51(7):1529–1585
Falke JJ, Bass RB, Butler SL, Chervitz SA, Danielson MA (1997) The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol 13:457–512
Fernö A, Pitcher TJ, Melle W, Nøttestad L, Mackinson S, Hollingworth C, Misund OA (1998) The challenge of the herring in the Norwegian sea: making optimal collective spatial decisions. Sarsia 83(2):149–167
Feynman RP (1972) Statistical mechanics: a set of lectures. W. A. Benjamin Inc, Reading
FitzGerald THB, Dolan RJ, Friston KJ (2014) Model averaging, optimal inference, and habit formation. Front Hum Neurosci 8(457):1–11
FitzGerald THB, Dolan RJ, Friston KJ (2015) Dopamine, reward learning, and active inference. Front Comput Neurosci 9(136):1–16
Fotopoulou A, Tsakiris M (2017) Mentalizing homeostasis: the social origins of interoceptive inference. Neuropsychoanalysis 19(1):3–28
Freddolino PL, Tavazoie S (2012) Beyond homeostasis: a predictive-dynamic framework for understanding cellular behavior. Annu Rev Cell Dev 28:363–384
Friston KJ (2002) Functional integration and inference in the brain. Prog Neurobiol 68(2):113–143
Friston KJ (2003) Learning and inference in the brain. Neural Netw 16(9):1325–1352
Friston KJ (2005) A theory of cortical responses. Philos Trans R Soc B 360(1456):815–836
Friston KJ (2008) Hierarchical models in the brain. PLoS Comput Biol 4(11):e1000211
Friston KJ (2009) The free-energy principle: a rough guide to the brain? Trends Cogn Sci 13(7):293–301
Friston KJ (2010) The free-energy principle: a unified brain theory? Nat Rev Neurosci 11(2):127–138
Friston KJ (2011) Embodied inference: or “I think therefore I am, if I am what I think”. In: Tschacher W, Bergomi C (eds) The implications of embodiment: cognition and communication. Imprint Academic, Exeter, pp 89–125
Friston KJ (2012a) A free energy principle for biological systems. Entropy 14(11):2100–2121
Friston KJ (2012b) Policies and priors. In: Gutkin B, Ahmed SH (eds) Computational neuroscience of drug addiction, Springer series in computational neuroscience 10. Springer, New York, pp 237–283
Friston KJ (2013) Life as we know it. J R Soc Interface 10(86):20130475
Friston KJ (2017) Self-evidencing babies: commentary on “Mentalizing homeostasis: the social origins of interoceptive inference” by Fotopoulou & Tsakiris. Neuropsychoanalysis 19(1):43–47
Friston KJ (2018) Am I self-conscious? (Or does self-organisation entail self-consciousness?). Front Psychol 9:579
Friston KJ, Ao P (2012) Free energy, value, and attractors. Comput Math Methods Med 937860
Friston KJ, Kiebel S (2009) Predictive coding under the free-energy principle. Philos Trans R Soc B 364(1521):1211–1221
Friston KJ, Stephan KE (2007) Free-energy and the brain. Synthese 159(3):417–458
Friston KJ, Kilner J, Harrison L (2006) A free energy principle for the brain. J Physiol Paris 100(1–3):70–87
Friston KJ, Mattout J, Trujillo-Barreto N, Ashburner J, Penny WD (2007) Variational free energy and the Laplace approximation. NeuroImage 34(1):220–234
Friston KJ, Daunizeau J, Kiebel SJ (2009) Reinforcement learning or active inference? PLoS ONE 4(7):e6421
Friston KJ, Daunizeau J, Kilner J, Kiebel SJ (2010a) Action and behavior: a free-energy formulation. Biol Cybern 102(3):227–260
Friston KJ, Stephan KE, Li B, Daunizeau J (2010b) Generalised filtering. Math Problems Eng 3:621670
Friston KJ, Adams RA, Montague R (2012a) What is value—accumulated reward or evidence? Front Neurorobot 6:11
Friston KJ, Adams RA, Perrinet L, Breakspear M (2012b) Perceptions as hypotheses: saccades as experiments. Front Psychol 3(151):1–20
Friston KJ, Breakspear M, Deco G (2012c) Perception and self-organized instability. Front Comput Neurosci 6(44):1–19
Friston KJ, Samothrakis S, Montague R (2012d) Active inference and agency: optimal control without cost functions. Biol Cybern 106(8–9):523–541
Friston KJ, Thornton C, Clark A (2012e) Free-energy minimization and the dark-room problem. Front Psychol 3:130
Friston KJ, Schwartenbeck P, FitzGerald T, Moutoussis M, Behrens T, Dolan RJ (2013) The anatomy of choice: active inference and agency. Front Hum Neurosci 7(598):1–18
Friston KJ, Levin M, Sengupta B, Pezzulo G (2015a) Knowing one’s place: a free-energy approach to pattern regulation. J R Soc Interface 12(20141383):1–12
Friston KJ, Rigoli F, Ognibene D, Mathys CD, Fitzgerald T, Pezzulo G (2015b) Active inference and epistemic value. Cogn Neurosci 6(4):187–224
Friston KJ, FitzGerald T, Rigoli F, Schwartenbeck P, O’Doherty J, Pezzulo G (2016) Active inference and learning. Neurosci Biobehav Rev 68:862–879
Friston KJ, FitzGerald T, Rigoli F, Schwartenbeck P, Pezzulo G (2017a) Active inference: a process theory. Neural Comput 29(1):1–49
Friston KJ, Lin M, Frith CD, Pezzulo G, Hobson JA, Ondobaka S (2017b) Active inference, curiosity and insight. Neural Comput 29(10):2633–2683
Friston KJ, Parr T, de Vries B (2017c) The graphical brain: belief propagation and active inference. Netw Neurosci 1(4):381–414
Friston KJ, Rosch R, Parr T, Price C, Bowman H (2017d) Deep temporal models and active inference. Neurosci Biobehav Rev 77:388–402
Friston KJ, Parr T, Zeidman P (2018) Bayesian model reduction. arXiv:1805.07092
Fuster JM (2001) The prefrontal cortex—an update: time is of the essence. Neuron 30(2):319–333
Fuster JM (2004) Upper processing stages of the perception–action cycle. Trends Cogn Sci 8(4):143–145
Gagliano M (2015) In a green frame of mind: perspectives on the behavioural ecology and cognitive nature of plants. AoB Plants 7:75
Gärdenfors P (1995) Cued and detached representations in animal cognition. Behav Proc 35:263–273
Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ (2017) Cardiovascular and autonomic reactivity to psychological stress: neurophysiological substrates and links to cardiovascular disease. Auton Neurosci Basic Clin 207:2–9
Giurfa M (2013) Cognition with few neurons: higher-order learning in insects. Trends Neurosci 36(5):285–294
Godfrey-Smith P (1995) Spencer and Dewey on life and mind. In: Boden MA (ed) The philosophy of artificial life, Oxford Readings in Philosophy, chapter 12. Oxford University Press, Oxford, pp 314–331
Godfrey-Smith P (1996) Complexity and the function of mind in nature. Cambridge Studies in Philosophy and Biology. Cambridge University Press, Cambridge
Godfrey-Smith P (2002) Environmental complexity and the evolution of cognition. In: Sternberg RJ, Kaufman JC (eds) The evolution of intelligence, Chapter 10. Lawrence Erlbaum Associates Inc, Mahwah, pp 223–250
Godfrey-Smith P (2016a) Individuality, subjectivity, and minimal cognition. Biol Philos 31(6):775–796
Godfrey-Smith P (2016b) Mind, matter, and metabolism. J Philos 113(10):481–506
Goodwin GM, McCloskey DI, Mitchell JH (1972) Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol 226(1):173–190
Grush R (2004) The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci 27(3):377–442
Gu X, Hof PR, Friston KJ, Fan J (2013) Anterior insular cortex and emotional awareness. J Comp Neurol 521(15):3371–3388
Hennessey TM, Rucker WB, McDiarmid CG (1979) Classical conditioning in paramecia. Anim Learn Behav 7(4):417–423
Heschl A (1990) L = C: a simple equation with astonishing consequences. J Theor Biol 145:13–40
Hobson JA, Friston KJ (2012) Waking and dreaming consciousness: neurobiological and functional considerations. Prog Neurobiol 98(1):82–98
Hohwy J (2013) The predictive mind. Oxford University Press, Oxford
Hohwy J (2016) The self-evidencing brain. Noûs 50(2):259–285
Hohwy J (2017a) How to entrain your evil demon. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 2. MIND Group, Frankfurt am Main, pp 1–15
Hohwy J (2017b) Priors in perception: top-down modulation, Bayesian perceptual learning rate, and prediction error minimization. Conscious Cogn 47:75–85
Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF (2005) Neural systems responding to degrees of uncertainty in human decision-making. Science 310(5754):1680–1683
Huang Y, Rao RPN (2011) Predictive coding. Wiley Interdiscip Rev Cogn Sci 2(5):580–593
Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML (2006) Neural signatures of economic preferences for risk and ambiguity. Neuron 49(5):765–775
Iodice P, Porciello G, Bufalari I, Barca L, Pezzulo G (2019) An interoceptive illusion of effort induced by false heart-rate feedback. Proc Natl Acad Sci 116(28):13897–13902
Kabadayi C, Osvath M (2017) Ravens parallel great apes in flexible planning for tool-use and bartering. Science 357(6347):202–204
Kahneman D, Tversky A (1979) Prospect theory: an analysis of decision under risk. Econometrica 47(2):263–292
Kalman RE (1960) A new approach to linear filtering and prediction problems. J Basic Eng 82(1):35–45
Kalman RE, Bucy RS (1961) New results in linear filtering and prediction theory. J Basic Eng 83(1):95–108
Kanai R, Komura Y, Shipp S, Friston KJ (2015) Cerebral hierarchies: predictive processing, precision and the pulvinar. Philos Trans R Soc B 370(1668):69–81
Kaplan R, Friston KJ (2018) Planning and navigation as active inference. Biol Cybern 112(4):323–343
Keramati M, Gutkin B (2014) Homeostatic reinforcement learning for integrating reward collection and physiological stability. eLife 3(e04811):1–26
Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport JS, Feinstein JS, Feusner JD, Garfinkel SN, Lane RD, Mehling WE, Meuret AE, Nemeroff CB, Oppenheimer S, Petzschner FH, Pollatos O, Rhudy JL, Schramm LP, Simmons WK, Stein MB, Stephan KE, Van Den Bergh O, Van Diest I, von Leupoldt A, Paulus MP (2018) Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 3:501–513
Kiebel SJ, Daunizeau J, Friston KJ (2008) A hierarchy of time-scales and the brain. PLoS Comput Biol 4(11):e1000209
Kirchhoff MD, Froese T (2017) Where there is life there is mind: in support of a strong life-mind continuity thesis. Entropy 19(4):169
Kirchhoff M, Parr T, Palacios E, Friston KJ, Kiverstein J (2018) The Markov blankets of life: autonomy, active inference and the free energy principle. J R Soc Interface 15(138):20170792
Knight FH (1921) Risk, uncertainty, and profit. Sentry Press, New York
Kozyreva A, Hertwig R (2019) The interpretation of uncertainty in ecological rationality. Synthese. https://doi.org/10.1007/s11229-019-02140-w
Kräuchi K, Wirz-Justice A (1994) Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 267(3 Pt 2):R819–R829
Krogh A, Lindhard J (1913) The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47:112–136
Krupenye C, Kano F, Hirata S, Call J, Tomasello M (2016) Great apes anticipate that other individuals will act according to false beliefs. Science 354(6308):110–114
Lee TS, Mumford D (2003) Hierarchical Bayesian inference in the visual cortex. J Opt Soc Am A 20(7):1434–1448
Lee D, McGreevy BP, Barraclough DJ (2005) Learning and decision making in monkeys during a rock–paper–scissors game. Cogn Brain Res 25(2):416–430
Levin M, Pezzulo G, Finkelstein JM (2017) Endogenous bioelectric signaling networks: exploiting voltage gradients for control of growth and form. Annu Rev Biomed Eng 19:353–387
Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW (2010) Neural representation of subjective value under risk and ambiguity. J Neurophysiol 103(2):1036–1047
Lewis D (1973a) Causation. J Philos 70(17):556–567
Lewis D (1973b) Counterfactuals. Basil Blackwell Ltd, Oxford
Lewis D (1979) Counterfactual dependence and time’s arrow. Noûs 13:455–476
Limanowski J, Friston KJ (2018) ‘Seeing the dark’: grounding phenomenal transparency and opacity in precision estimation for active inference. Front Psychol 9:643
Linson A, Clark A, Ramamoorthy S, Friston KJ (2018) The active inference approach to ecological perception: general information dynamics for natural and artificial embodied cognition. Front Robot AI 5:21
Lyon P (2015) The cognitive cell: bacterial behavior reconsidered. Front Microbiol 6:264
Lyon P (2019) Of what is “minimal cognition” the half-baked version? Adapt Behav 1–18
Mackie GO, Burighel P (2005) The nervous system in adult tunicates: current research directions. Can J Zool 83:151–183
Mathys CD, Lomakina EI, Daunizeau J, Iglesias S, Brodersen KH, Friston KJ, Stephan KE (2014) Uncertainty in perception and the hierarchical Gaussian filter. Front Hum Neurosci 8(825):1–24
Maturana HR, Varela FJ (1980) Autopoiesis and cognition: the realization of the living. D. Reidel Publishing Company, Dordrecht
McCoy JW (1977) Complexity in organic evolution. J Theor Biol 68(3):457–488
McEwen BS, Stellar E (1993) Stress and the individual: mechanisms leading to disease. Arch Intern Med 153(18):2093–2101
McGregor S, Baltieri M, Buckley CL (2015) A minimal active inference agent. arXiv:1503.04187
Menaker M, Murphy ZC, Sellix MT (2013) Central control of peripheral circadian oscillators. Curr Opin Neurobiol 23(5):741–746
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667
Metzinger T (2017) The problem of mental action: Predictive control without sensory sheets. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 19. MIND Group, Frankfurt am Main, pp 1–26
Mikhalevich I, Powell R, Logan C (2017) Is behavioural flexibility evidence of cognitive complexity? How evolution can inform comparative cognition. Interface Focus 7(3):20160121
Miracchi L (2019) A competence framework for artificial intelligence research. Philos Psychol. 32(5):588–633
Mirza MB, Adams RA, Mathys CD, Friston KJ (2016) Scene construction, visual foraging, and active inference. Front Comput Neurosci 10(56):1–16
Mitchell A, Romano GH, Groisman B, Yona A, Dekel E, Kupiec M, Dahan O, Pilpel Y (2009) Adaptive prediction of environmental changes by microorganisms. Nature 460(7252):220–224
Moore BR (2004) The evolution of learning. Biol Rev 79(2):301–335
Moran RJ, Symmonds M, Dolan RJ, Friston KJ (2014) The brain ages optimally to model its environment: evidence from sensory learning over the adult lifespan. PLoS Comput Biol 10(1):e1003422
Moreno A, Etxeberria A (2005) Agency in natural and artificial systems. Artif Life 11:161–175
Morgan A (2018a) Mindless accuracy: on the ubiquity of content in nature. Synthese 195(12):5403–5429
Morgan A (2018b) Pictures, plants, and propositions. Mind Mach 29(2):309–329
Morville T, Friston KJ, Burdakov D, Siebner HR, Hulme OJ (2018) The homeostatic logic of reward. bioRxiv
Mugan U, MacIver MA (2019) The shift from life in water to life on land advantaged planning in visually-guided behavior. bioRxiv
Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T (2005) Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308(5720):414–415
Neill WH (1979) Mechanisms of fish distribution in heterothermal environments. Am Zool 19(1):305–317
Nicolis G, Prigogine I (1977) Self-organization in nonequilibrium systems: From dissipative structures to order through fluctuations. Wiley, New York
Nute D (1975) Counterfactuals. Notre Dame J Formal Logic 16(4):476–482
Owens AP, Allen M, Ondobaka S, Friston KJ (2018) Interoceptive inference: from computational neuroscience to clinic. Neurosci Biobehav Rev 90:174–183
Palacios ER, Razi A, Parr T, Kirchhoff MD, Friston KJ (2020) On Markov blankets and hierarchical self-organisation. J Theor Biol 486:110089
Palmer CJ, Seth AK, Hohwy J (2015) The felt presence of other minds: predictive processing, counterfactual predictions, and mentalising in autism. Conscious Cogn 36:376–389
Parr T, Friston KJ (2017) Uncertainty, epistemics and active inference. J R Soc Interface 14(20170376):1–10
Parr T, Friston KJ (2018a) The anatomy of inference: generative models and brain structure. Front Comput Neurosci 12:90
Parr T, Friston KJ (2018b) The discrete and continuous brain: from decisions to movement–and back again. Neural Comput 30:1–29
Parr T, Corcoran AW, Friston KJ, Hohwy J (2019) Perceptual awareness and active inference. Neurosci Conscious 5(1):niz012
Paulus MP, Stein MB (2006) An insular view of anxiety. Biol Psychiat 60(4):383–387
Pavlov IP (1902) The work of the digestive glands. Charles Griffin & Co., Ltd, London
Payzan-LeNestour E, Bossaerts P (2011) Risk, unexpected uncertainty, and estimation uncertainty: Bayesian learning in unstable settings. PLoS Comput Biol 7(1):e1001048
Pearl J (1988) Probabilistic reasoning in intelligent systems: networks of plausible inference. Morgan Kaufmann Publishers, San Mateo
Penny W, Stephan K (2014) A dynamic Bayesian model of homeostatic control. Lect Notes Comput Sci 8779:60–69
Penny WD, Zeidman P, Burgess N (2013) Forward and backward inference in spatial cognition. PLoS Comput Biol 9(12):e1003383
Perry CJ, Barron AB, Cheng K (2013) Invertebrate learning and cognition: relating phenomena to neural substrate. Wiley Interdiscip Rev Cogn Sci 4(5):561–582
Peters A, McEwen BS, Friston KJ (2017) Uncertainty and stress: why it causes diseases and how it is mastered by the brain. Prog Neurobiol 156:164–188
Petzschner FH, Weber LAE, Gard T, Stephan KE (2017) Computational psychosomatics and computational psychiatry: toward a joint framework for differential diagnosis. Biol Psychiat 82:421–430
Pezzulo G (2008) Coordinating with the future: the anticipatory nature of representation. Mind Mach 18(2):179–225
Pezzulo G (2014) Why do you fear the bogeyman? An embodied predictive coding model of perceptual inference. Cogn Affect Behav Neurosci 14(3):902–911
Pezzulo G (2017) Tracing the roots of cognition in predictive processing. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 20. MIND Group, Frankfurt am Main, pp 1–20
Pezzulo G, Castelfranchi C (2007) The symbol detachment problem. Cogn Process 8(2):115–131
Pezzulo G, Castelfranchi C (2009) Thinking as the control of imagination: a conceptual framework for goal-directed systems. Psychol Res 73(4):559–577
Pezzulo G, Cisek P (2016) Navigating the affordance landscape: feedback control as a process model of behavior and cognition. Trends Cogn Sci 20(6):414–424
Pezzulo G, Rigoli F, Friston KJ (2015) Active inference, homeostatic regulation and adaptive behavioural control. Prog Neurobiol 134:17–35
Pezzulo G, Cartoni E, Rigoli F, Pio-Lopez L, Friston KJ (2016) Active inference, epistemic value, and vicarious trial and error. Learn Memory 23(7):322–338
Pezzulo G, Kemere C, van der Meer MAA (2017) Internally generated hippocampal sequences as a vantage point to probe future-oriented cognition. Ann N Y Acad Sci 1396(1):144–165
Pezzulo G, Rigoli F, Friston KJ (2018) Hierarchical active inference: a theory of motivated control. Trends Cogn Sci 22(4):294–306
Powers WT (1973) Feedback: beyond behaviorism. Science 179(4071):351–356
Preuschoff K, Quartz SR, Bossaerts P (2008) Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28(11):2745–2752
Quadt L, Critchley HD, Garfinkel SN (2018) The neurobiology of interoception in health and disease. Ann N Y Acad Sci 1428(1):112–128
Raby CR, Alexis DM, Dickinson A, Clayton NS (2007) Planning for the future by western scrub-jays. Nature 445(7130):919–921
Ramsay DS, Woods SC (2014) Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 121(2):225–247
Ramsay DS, Woods SC (2016) Physiological regulation: how it really works. Cell Metab 24(3):361–364
Ramstead MJD, Badcock PB, Friston KJ (2018) Answering Schrödinger’s question: a free-energy formulation. Phys Life Rev 24:1–16
Rao RPN, Ballard DH (1999) Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci 2(1):79–87
Read CR, Garnier S, Beekman M, Latty T (2015) Information integration and multiattribute decision making in non-neuronal organisms. Anim Behav 100:44–50
Redish AD (2016) Vicarious trial and error. Nat Rev Neurosci 17(3):147–159
Redshaw J, Bulley A (2018) Future-thinking in animals: Capacities and limits. In: Oettingen G, Sevincer AT, Gollwitzer PM (eds) The psychology of thinking about the future, Chapter 2. The Guilford Press, New York, pp 31–51
Requin J, Brener J, Ring C (1991) Preparation for action. In: Jennings JR, Coles MGH (eds) Handbook of cognitive psychophysiology: central and autonomic nervous system approaches, chapter 4. Wiley, New York, pp 357–448
Rust MJ, Markson JS, Lane WS, Fisher DS, O’Shea EK (2007) Ordered phosphorylation governs oscillation of a three-protein circadian clock. Science 318(5851):809–812
Sales AC, Friston KJ, Jones MW, Pickering AE, Moran RJ (2019) Locus coeruleus tracking of prediction errors optimises cognitive flexibility: an active inference model. PLoS Comput Biol 15(1):e1006267
Salman H, Libchaber A (2007) A concentration-dependent switch in the bacterial response to temperature. Nat Cell Biol 9(9):1098–1100
Sanchez-Fibla M, Bernardet U, Wasserman E, Pelc T, Mintz M, Jackson JC, Pennartz CMA, Verschure PFMJ (2010) Allostatic control for robot behavior regulation: a comparative rodent-robot study. Adv Compl Syst 13(3):377–403
Schacter DL, Addis DR (2007) The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc B Biol Sci 362(1481):773–786
Schrödinger E (1992) What is life? With “Mind and matter” and “Autobiographical sketches”. Cambridge University Press, Cambridge
Schulkin J, Sterling P (2019) Allostasis: a brain-centered, predictive mode of physiological regulation. Trends Neurosci 42(10):740–752
Schwartenbeck P, FitzGerald T, Dolan RJ, Friston KJ (2013) Exploration, novelty, surprise, and free energy minimization. Front Psychol 4(710):1–5
Schwartenbeck P, FitzGerald THB, Mathys CD, Dolan R, Kronbichler M, Friston KJ (2015) Evidence for surprise minimization over value maximization in choice behavior. Sci Rep 5(16575):1–14
Schwartenbeck P, Passecker J, Hauser TU, FitzGerald THB, Kronbichler M, Friston KJ (2019) Computational mechanisms of curiosity and goal-directed exploration. eLife 8:e41703
Segundo-Ortin M, Calvo P (2019) Are plants cognitive? A reply to Adams. Stud Hist Philos Sci 73:64–71
Seifert U (2012) Stochastic thermodynamics, fluctuation theorems and molecular machines. Rep Prog Phys 75(12):126001
Sengupta B, Stemmler MB, Friston KJ (2013) Information and efficiency in the nervous system—a synthesis. PLoS Comput Biol 9(7):e1003157
Seth AK (2013) Interoceptive inference, emotion, and the embodied self. Trends Cogn Sci 17(11):565–573
Seth AK (2014) A predictive processing theory of sensorimotor contingencies: explaining the puzzle of perceptual presence and its absence in synesthesia. Cogn Neurosci 5(2):97–118
Seth AK (2015) The cybernetic Bayesian brain: From interoceptive inference to sensorimotor contingencies. In: Metzinger T, Windt JM (eds) Open MIND. MIND Group, Frankfurt am Main, pp 1–24
Seth AK, Friston KJ (2016) Active interoceptive inference and the emotional brain. Philos Trans R Soc B 371(1708):1–10
Seth AK, Suzuki K, Critchley HD (2012) An interoceptive predictive coding model of conscious presence. Front Psychol 2(395):1–16
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27(3):379–423
Shipp S (2016) Neural elements for predictive coding. Front Psychol 7(1792):1–21
Shipp S, Adams RA, Friston KJ (2013) Reflections on agranular architecture: predictive coding in the motor cortex. Trends Cogn Sci 36(12):706–716
Smith GP (2000) Pavlov and integrative physiology. Am J Physiol Regul Integr Comp Physiol 279(3):R743–R755
Smith R, Thayer JF, Khalsa SS, Lane RD (2017) The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev 75:274–296
Smith-Ferguson J, Beekman M (2019) Who needs a brain? Slime moulds, behavioural ecology and minimal cognition. Adapt Behav. https://doi.org/10.1177/1059712319826537
Solway A, Botvinick MM (2012) Goal-directed decision making as probabilistic inference: a computational framework and potential neural correlates. Psychol Rev 119(1):120–154
Spencer H (1867) First principles, 2nd edn. Williams & Norgate, London
Spratling MW (2017) A review of predictive coding algorithms. Brain Cogn 112:92–97
Sprigge TLS (1970) Facts, words and beliefs. Routledge & Keegan Paul, London
Srinivasan MV, Laughlin SB, Dubs A (1982) Predictive coding: a fresh view of inhibition in the retina. Proc R Soc B 216(1205):427–459
Stalnaker RC (1968) A theory of conditionals. In: Rescher N (ed) Studies in logical theory, American Philosophical Quarterly supplementary monograph series. Basil Blackwell Ltd, Oxford, pp 98–112
Stanley ML, Stewart GW, De Brigard F (2017) Counterfactual plausibility and comparative similarity. Cogn Sci 41(Suppl 5):1216–1228
Steiner AP, Redish AD (2014) Behavioral and neurophysiological correlates of regret in rat decision-making on a neuroeconomic task. Nat Neurosci 17(7):995–1002
Stephan KE, Manjaly ZM, Mathys CD, Weber LAE, Paliwal S, Gard T, Tittgemeyer M, Fleming SM, Haker H, Seth AK, Petzschner FH (2016) Allostatic self-efficacy: a metacognitive theory of dyshomeostasis-induced fatigue and depression. Front Hum Neurosci 10(550):1–27
Sterelny K (2003) Thought in a hostile world: the evolution of human cognition. Blackwell Publishing, Malden
Sterling P (2004) Principles of allostasis: optimal design, predictive regulation, pathophysiology and rational therapeutics. In: Schulkin J (ed) Allostasis, homeostasis, and the costs of physiological adaptation, Chapter 1. Cambridge University Press, Cambridge, pp 17–64
Sterling P (2012) Allostasis: a model of predictive regulation. Physiol Behav 106(1):5–15
Sterling P, Eyer J (1988) Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J (eds) Handbook of life stress, cognition and health, Chapter 34. Wiley, New York, pp 629–649
Suddendorf T, Corballis MC (1997) Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr 123(2):133–167
Suddendorf T, Corballis MC (2007) The evolution of foresight: what is mental time travel, and is it unique to humans? Behav Brain Sci 30(3):299–313
Suddendorf T, Redshaw J (2017) Anticipation of future events. In: Vonk J, Shackelford TK (eds) Encyclopedia of animal cognition and behavior. Springer, Berlin
Suddendorf T, Bulley A, Miloyan B (2018) Prospection and natural selection. Curr Opin Behav Sci 24:26–31
Sweis BM, Thomas MJ, Redish AD (2018) Mice learn to avoid regret. PLoS Biol 16(6):e2005853
Tagkopoulos I, Liu Y-C, Tavazoie S (2008) Predictive behavior within microbial genetic networks. Science 320(5881):1313–1317
Tang SKY, Marshall WF (2018) Cell learning. Curr Biol 28(20):R1180–R1184
Teff KL (2011) How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav 103(1):44–50
Thompson E (2007) Mind in life: biology, phenomenology and the sciences of mind. Harvard University Press, Cambridge
Todd W (1964) Counterfactual conditionals and the presuppositions of induction. Philos Sci 31(2):101–110
Tschantz A, Seth AK, Buckley CL (2019) Learning action-oriented models through active inference. bioRxiv
Van de Cruys S (2017) Affective value in the predictive mind. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 24. MIND Group, Frankfurt am Main, pp 1–21
van Duijn M, Keijzer F, Franken D (2006) Principles of minimal cognition: casting cognition as sensorimotor coordination. Adapt Behav 14(2):157–170
Verschure PFMJ, Pennartz CMA, Pezzulo G (2014) The why, what, where, when and how of goal-directed choice: neuronal and computational principles. Philos Trans R Soc B 369(1655):20130483
Vincent P, Parr T, Benrimoh D, Friston KJ (2019) With an eye on uncertainty: modelling pupillary responses to environmental volatility. PLoS Comput Biol 15(7):e1007126
Wen Y, Zhou W, Zhu X, Cheng S, Xiao G, Li Y, Zhu Y, Wang Z, Wan C (2015) An investigation of circadian rhythm in Escherichia coli. Biol Rhythm Res 46(5):753–762
Wheeler M (2011) Mind in life or life in mind? Making sense of deep continuity. J Conscious Stud 18(5):148–168
Wiener N (1961) Cybernetics: Or control and communication in the animal and the machine, 2nd edn. MIT Press, Cambridge
Wiese W (2017) Action is enabled by systematic misrepresentations. Erkenntnis 82(6):1233–1252
Wiese W, Metzinger T (2017) Vanilla PP for philosophers: a primer on predictive processing. In: Metzinger T, Wiese W (eds) Philosophy and predictive processing, Chapter 1. MIND Group, Frankfurt am Main, pp 1–18
Williams D (2018) Predictive minds and small-scale models: Kenneth Craik’s contribution to cognitive science. Philos Explor 21(2):245–263
Williams D, Colling L (2018) From symbols to icons: the return of resemblance in the cognitive neuroscience revolution. Synthese 195(5):1941–1967
Yon D, de Lange FP, Press C (2019) The predictive brain as a stubborn scientist. Trends Cogn Sci 23(1):6–8
Zénon A, Solopchuk O, Pezzulo G (2018) An information-theoretic perspective on the costs of cognition. Neuropsychologia 123:5–18
Zwicker D, Lubensky DK, ten Wolde PR (2010) Robust circadian clocks from coupled protein-modification and transcription-translation cycles. Proc Natl Acad Sci 107(52):22540–22545
Acknowledgements
AWC is supported by an Australian Government Research Training Program (RTP) scholarship. JH is supported by the Australian Research Council (DP160102770, DP190101805). This research has received funding from the European Union’s Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2 to GP). We would like to thank participants at the Science of the Self research forum and the 22nd Annual Meeting of the Association for the Scientific Study of Consciousness for feedback on earlier presentations of this work. We also wish to thank Louise Kyriaki, Dan Williams, members of the Cognition & Philosophy Lab—especially Stephen Gadsby, Andy Mckilliam, Kelsey Perrykkad, and Iwan Williams—and two anonymous reviewers for insightful comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Corcoran, A.W., Pezzulo, G. & Hohwy, J. From allostatic agents to counterfactual cognisers: active inference, biological regulation, and the origins of cognition. Biol Philos 35, 32 (2020). https://doi.org/10.1007/s10539-020-09746-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10539-020-09746-2