Abstract
Cognitive neuroscientists are turning to an increasingly rich array of neurodynamical systems to explain mental phenomena. In these explanations, cognitive capacities are decomposed into a set of functions, each of which is described mathematically, and then these descriptions are mapped on to corresponding mathematical descriptions of the dynamics of neural systems. In this paper, I outline a novel explanatory schema based on these explanations. I then argue that these explanations present a novel type of dynamicism for the philosophy of mind and neuroscience, componential dynamicism, that focuses on the parts of cognitive systems that fill certain functional roles in producing cognitive phenomena.

Adapted from Kelso (1995, p. 57)

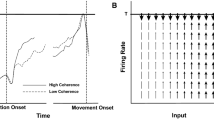
Adapted from Roitman and Shadlen (2002, p. 9482)


Similar content being viewed by others
Notes
For this essay, I adopt an object, property, and relation ontology. On such a view, objects are analyzed as bundles of properties with some substrate. This approach is adopted for convenience only, and the claims can be suitably recast in other terms. In order to cast a wide net, in this section I have framed the view in terms of individuals in the logical sense (cf. Strawson 1959). I thank a reviewer for pushing me on this point.
This distinction between dynamical systems and dynamical systems theory is often overlooked. I explore and defend this distinction elsewhere.
Systems can also evolve with respect to other variables besides time as noted, but to simplify I will often focus on time.
Some dynamicists might object to such a characterization of dynamical systems as ignoring parts of these systems. Specifically, Bechtel classifies connectionist models that refer to internal operations as dynamical systems that do refer to parts (Bechtel 1998). Bechtel says that “the question of how [dynamical] models comport with mechanistic explanatory objectives does not arise, since connectionist models are models of mechanisms. The [dynamicist] approach is employed by these theorists to analyze how these mechanisms behave” (Bechtel 1998, p. 312). This analysis involves decomposing the mechanism into parts and localizing the operations of the mechanism to the activity of these parts (Bechtel 1998; Bechtel and Richardson 2010). However, not all philosophers agree with this broad characterization of connectionism’s relation to dynamicism. For example, Zednik disagrees, arguing that “[i]nsofar as connectionist dynamical models do not… capture the dynamics of coupled brain-body-environment systems, but merely describe “internal” neural dynamics, they differ substantially” from other connectionist models that focus on “the behavior of the whole… brain-body-environment system” (Zednik 2008, pp. 1457–1458). These latter systems “can be understood as the interactive operations between two simpler subsystems: the agent’s motion M as the operation of subsystem A (the agent) on the one hand, and the sensory stimulus function S as the operation of subsystem E (the environment) on the other” (Zednik 2008, p. 1458). Notably, the behavior of these models is explained by an “understanding of the way in which individual parts of the system—the agent on the one hand and the environment on the other—interact” (Zednik 2009, p. 2301). There is thus some debate as to the appropriate interpretation of certain connectionist systems, and some such systems do refer to parts or parts of parts while others do not. My componential dynamicism is consistent with connectionist approaches that focus on internal dynamics.
Kelso himself would probably endorse parts, seeing as his research program includes both understanding how the neural mechanisms give rise to the dynamics captured by the HKB model and applying the lessons gleaned from dynamical analysis of behavioral phenomena to the brain. I do not have space to fully address Kelso’s own dynamicism (Kelso 1995), which is a remarkably deep and distinct application of dynamical ideas to analyzing cognitive phenomena and which should also be seen as an exception to the systemic approach. Kelso’s view is that there are dynamical laws that underlie many complex systems, including cognitive ones, and that cognitive phenomena result from these laws. In addition to this nomological component, his view has a mechanistic component, seeing the dynamical systems as mechanistic parts. For the time being, suffice to say that Kelso is not a target of my criticism.
From now on, I will exclusively use the term ‘parts’ to refer to subsubsystems of the system.
See below, “Functional neurodynamics” section, for a lengthier discussion of functions and componential dynamical systems.
Note that the parts of the fingers like their nerves or muscles do not necessarily determine the relative phase. Usually, those properties will determine the fingers’ movements. But there could be ways of setting the value for the relative phase other than the musculature, nerves, and other physiological parts that typically cause finger movements. Imagine, for example, that a mechanical device is used to oscillate the fingers, mimicking the behavioral output of the usual physical parts. On systemic dynamicism, this mechanical system is described in the same way as when the musculature and nerves control the movements. Systemic dynamicism cannot distinguish between the two systems, one organically controlled, the other artificially, because the role of the parts is excluded by systemic dynamicism. The inclusion of a function describing the motive forces on the fingers could distinguish between different sources of movement. Such a description will distinguish different sources of movement unless the proximal causes of the finger movements—viz., muscles and nerves versus artificial means—have precisely the same force functions in all contexts. But even then, the equations corresponding to such functions are interpreted; so the variables refer to those proximal causes, which will be organic in the first case and artificial in the second.
This division of types of dynamical systems is not meant to be a stark contrast. There can be systems with mixed dynamical descriptions. A car can be described systemically, componentially, or in a mixed fashion, with some terms for its environment and systemic properties and other terms for the properties of the parts of the car and environment. Thus, the two types of dynamical description stand at extremes on a scale of dynamical descriptions, with most such systems lying somewhere in the midst of the spectrum.
From this point on, I will focus on changes in properties, but this should not be read as excluding changes in objects or relations as underlying dynamical properties.
In fact, neurophysiological systems implement dynamical systems in a technical sense that I explore in other work. For the time being, instantiation here should be understood in the sense of token identity, so that if a neuron instantiates a dynamical system, then a subset of the neuron’s dynamical properties are an instance of the dynamical system; if a neural population instantiates a dynamical system, then a subset of the population’s dynamical properties are an instance of the dynamical system; etc. In addition, the neuron, neural population, etc. are themselves dynamical systems.
See Gold and Shadlen (2007) for extensive discussion of this research. Note that many aspects of this case are still actively researched. In particular, whether neurons in area LIP truly exhibit the integrate-to-bound dynamics discussed below is hotly debated (Latimer et al. 2015). For my present purposes, the fact that the details are still not settled does not matter, as I am merely illustrating how such explanations are constructed.
When describing this state change mathematically, both the smooth and non-saltatory nature of the change is captured by a continuous mathematical function. The mathematical function does not contain jump or point discontinuities, corresponding to a lack of saltatory changes in the system. The function is also continuously differentiable, corresponding to a lack of abrupt changes in the system. While there is also typically some monotonicity in the trajectories, the trajectory’s evolution is correlated with some input such that if the input changes sign, then the trajectory can switch from an increase to a decrease or vice versa. A description of a system’s evolution wherein the system resides in the same state despite changes in some variable does not count as an example of integration.
In other work, I intend to present a deeper analysis of functions.
Causal functions may be clearest example of compositional functions. Constitution is taken to be distinct from causation for a number of reasons, including that the former is synchronic and the latter diachronic. Whether a component of a physical system can help constitute that system without causal powers is an interesting open question, though this has recently been argued by a range of philosophers (see e.g. Huneman 2017 or Chirimuuta 2017).
Here, I deliberately include both causal and constitutive roles for the part in c-functions.
This model is often dubbed the drift diffusion model for historical reasons.
The system could also run out of evidence (e.g., if the dot display disappears) or some other stopping criterion could be met that results in a decision.
Carandini and Wang both discuss the neurons as computing an exponentiation function (Carandini and Heeger 2012; Wang 2002). In a case of pure exponential growth, for a given strength of evidence, the relative change in the firing rate, or the change in firing rate divided by the current rate, is constant (Stewart 2011). However, upon removal of the stimulus, LIP neurons will not maintain their firing rates indefinitely, and adding state-dependent leak and recurrent excitation exponential decay terms captures this decay (Usher and McClelland 2001; Wang 2002). Furthermore, the integration is noisy, requiring the addition of a noise term. Thus, neurons such as those in area LIP are ‘noisy leaky integrators’; their firing rate is a function not only of the change in firing but also contains a noise term and a loss (or ‘leak’) term (e.g. see Usher and McClelland 2001 or Wang 2002; for a philosophical discussion, see Eliasmith 2013, p. 43ff). The leak is dependent upon the state of the system, and so the internal system dynamics contribute to the state of the system. Wang’s model is even more complex but in short contains an additional recurrent excitation term that is driven by the state of the system. These noise and leak terms effectively curtail the integrative growth function as well as enforcing a biologically plausible decay in the firing rate at some time constant absent any driving force, while the differences in the strength of evidence correspond to differences in the relative growth rate constant driving the neuron’s activity.
Exactly how many steps depends on the number of subcapacities of the capacity. The set of subcapacities often implies some ordering to their execution. Some subsets of subcapacities may be executed serially or in parallel, whereas other subsets must be executed seriatim.
Does the part need to be causally active in the cognitive system? I will remain neutral on this issue herein; some explanations of cognitive capacities might reflect purely structural features of systems such that the parts’ causal powers are not relevant (see e.g. Chirimuuta 2017 or Huneman 2017). In the examples herein, causality is important, but I do not want to beg any questions about other cognitive phenomena.
For this discussion, the part of the brain, area LIP, is a subsubsystem because the subsystem is the brain itself.
Perhaps these processes should not be considered cognitive. I construe the term ‘cognitive’ quite widely, so I set aside this concern.
The computations executed by these neural dynamics are often magnitude to magnitude transformations or functions on magnitudes. I note that these computations are not obviously like the representational transformations posited by classical computational theories like the language of thought (Fodor 1975).
Hence, showing that e.g. a generalized linear model containing some independent variable can significantly capture some of the variance in neuronal firing rates is insufficient to show that the neuron’s function is to signal that variable. In order to argue that the neuron’s function is in part to signal the variable, there must be at least the additional step of modeling the neuronal dynamics, such as by fitting a Gaussian tuning curve.
Despite instantiating the same dynamical system, the physiological systems are different in LIP and ACC. In the case of LIP and the RDMT, integrative activity occurring on the timescale of hundreds of milliseconds during a single trial is instantiated by an increase in firing by individual neurons. In the case of ACC and the patch foraging task, integrative activity occurring on the timescale of tens of seconds over many trials is instantiated by an increase in the peak activity of individual neurons during a trial. These distinct timecourses indicate distinct physiological mechanisms instantiating the integrate-to-bound system. Furthermore, the integrate-to-bound in LIP is instantiated via a noisy but continuous-like increase in firing rates, whereas the integrate-to-bound in ACC is instantiated in a series of discontinuous transient increases in firing rates. These distinct firing rate patterns also indicate distinct physiological mechanisms. These two differences suggest that distinguishing between the dynamical system and the physical mechanism that possesses the dynamics is necessary to understand how cognitive neurobiologists go about explaining cognitive phenomena. In addition, the differences in the integrative activity will be reflected in models that include greater neuronal details. More abstract models, however, that focus just on the integrating peaks of activity may still use the same mathematical operations as used to describe the integrate-to-bound activity in area LIP.
This will also illustrate how the schema applies well beyond decision making to a range of cognitive capacities.
I cannot do justice to the two decades long research project, and the dozens of studies, that have gone into the development of the DN model of attention. An excellent review is in Reynolds and Heeger (2009).
The basic mathematical description of divisive normalization is
\( R = \gamma \frac{{D_{j}^{n} }}{{\sigma^{n} + \mathop \sum \nolimits_{k} D_{k}^{n} }} \)
for response of a cell R, input to the jth cell Dnj, and normalization pool summed over the normalization input Dnk (Carandini and Heeger 2012, p. 54). The parameters γ, n, and σ are fit to the data, with σ controlling how quickly the firing of a cell reaches its maximum. When σ is very large, the normalizing input has little effect on the firing rate, and when σ ~ 0, the input is largely determined by the normalizing pool of activity. If σ is roughly equivalent to the normalization input Dnk, the cell is only moderately determined by the activity of the normalization pool.
Some models do contain such parameterizations. What’s key here is that such models need not; and, as the illustration above showed, certain central cases of explanation of cognitive phenomena by neurodynamics do not.
References
Aljadeff J, Lansdell BJ, Fairhall AL, Kleinfeld D (2016) Analysis of neuronal spike trains, deconstructed. Neuron 91(2):221–259
Anderson JR (1990) The adaptive character of thought. Psychology Press, London
Anderson ML (2010) Neural reuse: a fundamental organizational principle of the brain. Behav Br Sci 33(04):245–266
Andersen R, Mountcastle V (1983) The influence of the angle of gaze upon the excitability of the light- sensitive neurons of the posterior parietal cortex. J Neurosci 3(3):532–548
Bailey TN (1993) The African leopard: ecology and behavior of a solitary felid. Columbia University Press, New York
Barack DL, Platt ML (2017) Engaging and exploring: cortical circuits for adaptive foraging decisions. In: Stevens JR (ed) Impulsivity. Springer, Cham, pp 163–199
Barack DL, Chang SWC, Platt ML (2017) Posterior cingulate neurons dynamically signal decisions to disengage during foraging. Neuron 96(2):339–347.e335
Bechtel W (1998) Representations and cognitive explanations: assessing the dynamicist’s challenge in cognitive science. Cognit Sci 22(3):295–318
Bechtel W, Richardson R (2010) Discovering complexity. MIT Press, Cambridge
Beer RD (1995) A dynamical systems perspective on agent-environment interaction. Artif Intell 72(1):173–215
Beer RD (2000) Dynamical approaches to cognitive science. Trends Cognit Sci 4(3):91–99
Bergeron V (2007) Anatomical and functional modularity in cognitive science: shifting the focus. Philos Psychol 20(2):175–195
Britten KH, Newsome WT (1998) Tuning bandwidths for near-threshold stimuli in area MT. J Neurophysiol 80(2):762–770
Britten KH, Shadlen MN, Newsome WT, Movshon JA (1992) The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12(12):4745–4765
Britten KH, Shadlen MN, Newsome WT, Movshon JA (1993) Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci 10(6):1157–1169
Carandini M, Heeger DJ (2012) Normalization as a canonical neural computation. Nat Rev Neurosci 13(1):51–62
Carandini M, Heeger DJ, Movshon JA (1997) Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci 17(21):8621–8644
Cavanaugh JR, Bair W, Movshon JA (2002) Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol 88(5):2530–2546
Charnov EL (1976) Optimal foraging, the marginal value theorem. Theor Popul Biol 9(2):129–136
Chemero A (2011) Radical embodied cognitive science. MIT Press, Cambridge
Chemero A, Silberstein M (2008) After the philosophy of mind: replacing scholasticism with science. Philos Sci 75(1):1–27
Chirimuuta M (2017) Explanation in computational neuroscience: causal and non-causal. Br J Philos Sci 69:849–880
Clark A (1980) Psychological models and neural mechanisms. Oxford University Press, Oxford
Clark A (1997) Being there: putting brain, body, and world together again. MIT Press, Cambridge, MA
Clark A (2000) Mindware: an introduction to the philosophy of cognitive science. Oxford University Press, Oxford
Clark A (2013) Mindware: an introduction to the philosophy of cognitive science. Oxford University Press Inc, Oxford
Craver CF (2007) Explaining the brain. Oxford University Press, Oxford
Craver CF, Darden L (2013) In search of mechanisms: discoveries across the life sciences. University of Chicago Press, Chicago
Cummins R (1975) Functional analysis. J Philos 72(20):741–765
Dennett DC (1981) Brainstorms: philosophical essays on mind and psychology. MIT Press, Cambridge
Eliasmith C (2013) How to build a brain: a neural architecture for biological cognition. Oxford University Press, Oxford
Fodor JA (1975) The language of thought. Harvard University Press, Cambridge
Giunti M (1997) Computation, dynamics, and cognition. Oxford University Press, Oxford
Gold JI, Shadlen MN (2000) Representation of a perceptual decision in developing oculomotor commands. Nature 404(6776):390–394
Gold JI, Shadlen MN (2007) The neural basis of decision making. Annu Rev Neurosci 30:535–574
Green S, Levy A, Bechtel W (2015) Design sans adaptation. Eur J Philos Sci 5(1):15–29
Grush R (1997) Yet another design for a brain? Review of Port and van Gelder. Mind Motion Philos Psychol 10:233–242
Haken H, Kelso JS, Bunz H (1985) A theoretical model of phase transitions in human hand movements. Biol Cybern 51(5):347–356
Hayden BY, Pearson JM, Platt ML (2011) Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci 14(7):933–939
Heeger DJ (1992) Normalization of cell responses in cat striate cortex. Vis Neurosci 9(2):181–197
Heuer HW, Britten KH (2002) Contrast dependence of response normalization in area MT of the Rhesus Macaque. J Neurophysiol 88:3398–3408
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80(3):953–978
Horgan T, Tienson J (1992) Cognitive systems as dynamical systems. Topoi 11(1):27–43
Horgan T, Tienson J (1994) A nonclassical framework for cognitive science. Synthese 101(3):305–345
Horgan T, Tienson J (1996) Connectionism and the philosophy of psychology. MIT Press, Cambridge
Huneman P (2017) Outlines of a theory of structural explanations. Philos Stud 175:1–38
James W (1890/1950) The principles of psychology. Dover Publications, New York
Jenny D (1996) Spatial organization of leopards Panthera pardus in Taï National Park, Ivory Coast: is rainforest habitat a ‘tropical haven’? J Zool 240(3):427–440
Jenny D, Zuberbühler K (2005) Hunting behaviour in West African forest leopards. Afr J Ecol 43(3):197–200
Kacelnik A, Vasconcelos M, Monteiro T, Aw J (2011) Darwin’s “tug-of-war” vs. starlings’ “horse-racing”: how adaptations for sequential encounters drive simultaneous choice. Behav Ecol Sociobiol 65(3):547–558
Kaplan DM (2011) Explanation and description in computational neuroscience. Synthese 183(3):339–373
Kaplan DM, Craver CF (2011) The explanatory force of dynamical and mathematical models in neuroscience: a mechanistic perspective. Philos Sci 78(4):601–627
Katz LN, Yates JL, Pillow JW, Huk AC (2016) Dissociated functional significance of decision-related activity in the primate dorsal stream. Nature 535(7611):285–288
Kelso JAS (1995) Dynamic patterns: the self-organization of brain and behavior. MIT Press, Cambridge
Kiyonaga A, Egner T (2016) Center-surround inhibition in working memory. Curr Biol 26(1):64–68
Kolling N, Behrens TEJ, Mars RB, Rushworth MFS (2012) Neural mechanisms of foraging. Science 336(6077):95–98
Kolling N, Wittmann MK, Behrens TE, Boorman ED, Mars RB, Rushworth MF (2016) Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci 19(10):1280–1285
Lamb M, Chemero A (2014) Structure and application of dynamical models in cognitive science. In: Proceedings of the annual meeting of the cognitive science society
Latimer KW, Yates JL, Meister ML, Huk AC, Pillow JW (2015) Single-trial spike trains in parietal cortex reveal discrete steps during decision-making. Science 349(6244):184–187
Levy A, Bechtel W (2013) Abstraction and the organization of mechanisms. Philos Sci 80(2):241–261
Louie K, Grattan LE, Glimcher PW (2011) Reward value-based gain control: divisive normalization in parietal cortex. J Neurosci 31(29):10627–10639
Lycan WG (1981) Form, function, and feel. J Philos 78(1):24–50
Maunsell JH, Van Essen DC (1983) Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 49(5):1127–1147
Mazurek ME, Roitman JD, Ditterich J, Shadlen MN (2003) A role for neural integrators in perceptual decision making. Cereb Cortex 13(11):1257–1269
McAdams CJ, Maunsell JH (1999) Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron 23(4):765–773
Mink JW (1996) The Basal Ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425
Mink JW (2003) The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 60(10):1365–1368
Neander K (2017) A mark of the mental: in defense of informational teleosemantics. MIT Press, Cambridge
Newell A (1980) Physical symbol systems. Cognit Sci 4(2):135–183
Paninski L, Pillow JW, Simoncelli EP (2004) Maximum likelihood estimation of a stochastic integrate-and-fire neural encoding model. Neural Comput 16(12):2533–2561
Piccinini G (2015) Physical computation: a mechanistic account. OUP, Oxford
Platt ML, Glimcher PW (1999) Neural correlates of decision variables in parietal cortex. Nature 400(6741):233–238
Port RF, van Gelder T (1995) Mind as motion: explorations in the dynamics of cognition. MIT Press, Cambridge
Pour-El MB (1974) Abstract computability and its relation to the general purpose analog computer (some connections between logic, differential equations and analog computers). Trans Am Math Soc 199:1–28
Putnam H (1967) The mental life of some machines. In: Castaneda H-N (ed) Intentionality, minds and perception. Wayne State University Press, Detroit
Reynolds JH, Heeger DJ (2009) The normalization model of attention. Neuron 61(2):168–185
Roitman JD, Shadlen MN (2002) Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22(21):9475–9489
Salzman CD, Britten KH, Newsome WT (1990) Cortical microstimulation influences perceptual judgements of motion direction. Nature 346(6280):174–177
Shadlen MN, Newsome WT (2001) Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86(4):1916–1936
Shadlen MN, Kiani R, Newsome WT, Gold JI, Wolpert DM, Zylberberg A, Ditterich J, de Lafuente V, Yang T, Roitman J (2016) Comment on “Single-trial spike trains in parietal cortex reveal discrete steps during decision-making”. Science 351(6280):1406–1406
Shapiro LA (2013) Dynamics and cognition. Mind Mach 23(3):353–375
Shenhav A, Straccia MA, Cohen JD, Botvinick MM (2014) Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci 17(9):1249–1254
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Stepp N, Chemero A, Turvey MT (2011) Philosophy for the rest of cognitive science. Top Cognit Sci 3(2):425–437
Stewart J (2011) Single variable calculus: early transcendentals. Brooks/Cole Publishing Company, Pacific Grove
Strait CE, Blanchard TC, Hayden BY (2014) Reward value comparison via mutual inhibition in ventromedial prefrontal cortex. Neuron 82(6):1357–1366
Strawson PF (1959) Individuals. Methuen, London
Strogatz S (2001) Nonlinear dynamics and chaos: with applications to physics, biology, chemistry, and engineering (studies in nonlinearity). Westview Press, Boulder
Treue S, Martinez Trujillo JC (1999) Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399(6736):575–579
Usher M, McClelland JL (2001) The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev 108(3):550
van Gelder T (1995) What might cognition be, if not computation? J Philos 92(7):345–381
Walmsley J (2008) Explanation in dynamical cognitive science. Minds Mach 18(3):331–348
Wang XJ (2002) Probabilistic decision making by slow reverberation in cortical circuits. Neuron 36(5):955–968
Wang XJ (2008) Decision making in recurrent neuronal circuits. Neuron 60(2):215–234
Wheeler M (2005) Reconstructing the cognitive world: the next step. MIT Press, Cambridge
Young MP, Hilgetag CC, Scannell JW (2000) On imputing function to structure from the behavioural effects of brain lesions. Philos Trans R Soc Lond B Biol Sci 355(1393):147–161
Zednik C (2008) Dynamical models and mechanistic explanations. In: Proceedings of the 30th annual conference of the cognitive science society
Zednik C (2009) The varieties of dynamicism. In: Proceedings of the 31st annual conference of the cognitive science society, pp 2298–2303
Zednik C (2011) The nature of dynamical explanation. Philos Sci 78(2):238–263
Zednik C (2015) Heuristics, descriptions, and the scope of mechanistic explanation. Explanation in biology. Springer, Cham, pp 295–318
Zeki SM (1974) Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J Physiol 236(3):549
Zeki S (1991) Cerebral akinetopsia (visual motion blindness). Brain 114(2):811–824
Zuberbühler K, Jenny D (2002) Leopard predation and primate evolution. J Hum Evol 43(6):873–886
Zuberbühler K, Jenny D, Bshary R (1999) The predator deterrence function of primate alarm calls. Ethology 105(6):477–490
Acknowledgements
This work has benefitted greatly from a number of comments over many years. The work draws on my dissertation at Duke University, and I would like to thank Walter Sinnott-Armstrong, Alex Rosenberg, Karen Neander, Felipe De Brigard, and Michael Platt for their thoughtful input. I would also like to thank Gaultiero Piccinini, Chris Peacocke, William Lycan, Ann-Sophie Barwich, Jorge Morales, Nemira Gasiunas, Michael Weisberg, and the University of Pennsylvania philosophy of science reading group for critical comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barack, D.L. Mental machines. Biol Philos 34, 63 (2019). https://doi.org/10.1007/s10539-019-9719-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10539-019-9719-6