Abstract
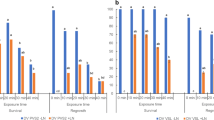
This study was carried out on Kober 5BB (Vitis Berlandieri × V. riparia) grape rootstock shoot tips during the preparatory steps preceding the direct immersion in liquid nitrogen, in order to overcome until now unsuccessful cryopreservation with this species. The exposure of shoot tips to 0.3–0.4 M sucrose leads to a high cell solute concentration. The treatment with plant vitrification solution (PVS2) alone, i.e., not followed by storage in liquid nitrogen, markedly affected shoot tip survival. After a 30 min exposure, regrowth percentage of shoot tips decreased from 94 % (control) to 57 %, and dropped to 15 % when the treatment was prolonged up to 60 min. After a 90 min exposure, no regrowth occurred. In addition, plantlets regenerated from shoot tips which underwent 60 min or more exposure to PVS2 showed signs of malformation. Microscope observations of shoot tips treated with 0.3 or 0.4 M sucrose and 30 min PVS2 showed the presence of cells starting to plasmolyze, localized in the area surrounding the apical meristem. A limited presence of starch grains in meristem and bract cells was also noted. However, the most conspicuous consequence of prolonged PVS2 treatment was convex plasmolysis. The phenomenon was dependent on the time of PVS2 exposure. Indeed, after a 30 min treatment, plasmolysis was minimal or absent, but it increased with longer exposure to PVS2 at 4 °C.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- CP:
-
cryoprotectant
- MS:
-
Murashige and Skoog medium
- DMSO:
-
dimethylsulfoxide
- LN:
-
liquid nitrogen
- PVS2:
-
plant vitrification solution No. 2
References
Attree, S.M., Sheffield, E.: Plasmolysis of Pteridium protoplasts: a study using light and scanning electron microscopy. — Planta 165: 151–157, 1985.
Drake, G.A., Carr, D.J., Anderson, W.P.: Plasmolysis, plasmodesmata, and the electrical coupling of oat coleoptile cells. — J. exp. Bot. 29: 1205–1214, 1978.
Engelmann, F.: In vitro conservation methods. — In: Ford-Lloyd, B.V., Newburry, H.J., Callow, J.A. (ed.): Biotechnology and Plant Genetic Resources, Conservation and Use. Pp. 119–162. CAB International, Wallingford 1997.
Engelmann, F.: Plant cryopreservation: progress and prospects. — In Vitro cell. dev. Biol. Plant 40: 427–433, 2004.
Fabbri, A., Ganino, T., Lombardi, M., Nisi, R.: [Cryopreservation of rootstock Kober 5BB (Vitis Berlandieri × Vitis riparia): anatomical aspects.]. — Italus Hortus 14: 82–86, 2007. [In Ital., ab: E.]
Lambardi, M., De Carlo, A.: Application of tissue culture to the germplasm conservation of temperate broad-leaf trees. — In: Jain, S.M., Ishii, K. (ed.): Micropropagation of Woody Trees and Fruits. Pp. 815–840. Kluwer Academic Publishers, Dordrecht 2003.
Lambardi, M., Fabbri, A., Caccavale, A.: Cryopreservation of white poplar (Populus alba L.) by vitrification of in vitrogrown shoot tips. — Plant Cell. Rep. 19: 213–218, 2000.
Lambardi, M., Lynch, P.T., Benelli, C., Mehra, A., Siddika, A.: Towards the cryopreservation of olive germplasm. — Adv. hort. Sci. 16: 165–174, 2002.
Levitt, J.: Plasmolysis shape in relation to freeze hardening of cabbage (Brassica oleracea cv. Early Jersey Wakefield) plants and to the effect of penetrating solutes. — Plant Cell Environ. 6: 465–470, 1983.
Lynch, P.T., Siddika, A., Mehra, A., Fabbri, A., Benelli, C., Lambardi, M.: The challenge of successful cryopreservation of olive (Olea europaea L.) shoot tips. — Adv. hort. Sci. 21: 211–214, 2007.
Matsumoto, T., Sakai, A.: Cryopreservation of axillary shoot tips of in vitro-grown grape (Vitis) by a two step vitrification protocol. — Euphytica 131: 299–304, 2003.
Matsumoto, T., Sakai, A., Takahashi, C., Yamada, K.: Cryopreservation of apical meristems of wasabi (Wasabia japonica) by encapsulation — vitrification method. — CryoLetters 247: 125–142, 1995.
Murashige, T., Skoog, F.A.: Revised medium for rapid growth and bioassay with tobacco tissue culture. — Physiol. Plant. 15: 473–497, 1962.
Oparka, K.J.: Plasmolysis — new insights into an old process. — New Phytol. 126: 571–591, 1994.
Panis, B., Lambardi, M.: Status of cryopreservation technologies in plants (crops and forest trees). — In: Ruane, J., Sonnino, A., (ed.): The Role of Biotechnology in Exploring and Protecting Agricultural Genetic Resources. Pp. 61–78. FAO, Rome 2006.
Plessis, P.C., Leddet, C., Colas, A., Dereuddre, J.: Cryopreservation of Vitis vinifera L. cv. Chardonnay shoot tips by encapsulation-dehydration: effect of pretreatment, cooling and postculture conditions. — CryoLetters 14: 309–320, 1993.
Plessis, P.C., Leddet, C., Dereuddre, J.: Resistance to dehydration and to freezing in liquid nitrogen of alginate coated shoot tips of grapevine (Vitis vinifera L. cv. Chardonnay). — Compt. rend. Acad. Sci. Paris 313: 373–380, 1991.
Reed, B.: Plant Cryopreservation: A Practical Guide. — Springer, New York 2008.
Ruan, Y.L., Xu, S.M., White, R., Furbanck, R.T.: Genotypic and developmental evidence for the role of plasmodesmatal regulation in cotton fiber elongation mediated by callose turnover. — Plant Physiol. 136: 4104–4113, 2004.
Ruzin, S.: Plant Microtechnique and Microscopy. — Oxford University Press, Oxford 1999.
Sakai, A., Engelmann, F.: Vitrification, encapsulation-vitrification and droplet vitrification: a review. — CryoLetters 28: 151–172, 2007.
Sakai, A., Hirai, D., Niino, T.: Development of PVS-based vitrification and encapsulation-vitrification protocols. — In: Reed, B.M. (ed.). Plant Cryopreservation. A Practical Guide. Pp. 33–58. Springer, New York 2008.
Sakai, A., Kobayashi, S., Oiyama, I.: Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. — Plant cell. Rep. 9: 30–33, 1990.
Škrlep, K., Bergant, M., De Winter, G.M., Bohanec, B., Žel, J., Verpoorte, R., Van Iren, F., Camloh, M.: Cryopreservation of cell suspension cultures of Taxus × media and Taxus floridana. — Biol. Plant. 52: 329–333, 2008.
Tao, D., Li, P.H.: Classification of plant cell cryoprotectants. — J. theor. Biol. 123: 305–310, 1986.
Torelli, A., Bottura, C., Corradi, M., Branca, C.: TU92, a putative MIP gene involved in the early phases of “in vitro” culture and differentially switched off by hormonal treatments. — Biol. Plant. 42(Suppl.): S59, 1999.
Volk, G.M., Caspersen, A.M.: Plasmolysis and recovery of different cell types in cryoprotected shoot tips of Mentha piperita. — Protoplasma 231: 215–226, 2007.
Withers, L.A.: The cryopreservation of higher plant tissue and cell cultures — an overview with some current observations and future thoughts. — CryoLetters 1: 239–250, 1980.
Zhao, C., Wu, Y., Engelmann, F., Zhou, M.: Cryopreservation of axillary buds of grape (Vitis vinifera) in vitro plantlets. — CryoLetters 22: 321–328, 2001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganino, T., Silvanini, A., Beghé, D. et al. Anatomy and osmotic potential of the Vitis rootstock shoot tips recalcitrant to cryopreservation. Biol Plant 56, 78–82 (2012). https://doi.org/10.1007/s10535-012-0019-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-012-0019-0