Abstract
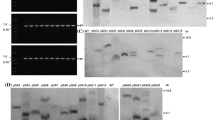
The shrub Chimonanthus praecox L. (wintersweet) which is native to Chinese montane forests produces its flowers in the midst of winter. This indicates that the floral organs of this species are adapted to growth and development under freezing temperatures. Here, we report the isolation and preliminary characterisation of a 33 kDa apoplastic antifreeze chitinase (CpCHT1) from the petals and its corresponding cDNA. The chitinase activity of CpCHT1 was confirmed by activity staining. Antifreeze activity was validated in terms of the formation of bipyramidal ice crystals and high thermal-hysteresis values. CpCHT1 was also found to affect the germination of fungal spores of four major plant pathogens. In addition, the gene and protein are expressed constitutively not only in flowers, but also in leaves, bark and root tissues. From these data we hypothesize that this protein is multifunctional and may protect wintersweet from freezing injury and provide nonspecific disease resistance.
Similar content being viewed by others
Abbreviations
- AFPs:
-
antifreeze proteins
- PDA:
-
potato dextrose agar
- PR:
-
pathogenesis-related proteins
- CLPs:
-
chitinase-like proteins
References
Antikainen, M., Griffith, M.: Antifreeze protein accumulation in freezing-tolerant cereals. — Physiol. Plant. 99: 423–432, 1997.
Atici, O., Nalbantoglu, B.: Antifreeze proteins in plants. — Phytochemistry 64: 1187–1196, 2003.
Bradford, M.M.: A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. — Anal. Biochem. 72: 1105–1112, 1976.
Bravo, L.A., Griffith, M.: Characterization of antifreeze activity in Antarctic plants. — J. exp. Bot. 56: 1189–1196, 2005.
DeVries, A.L.: Antifreeze peptides and glycopeptides in cold-water fishes. — Annu. Rev. Plant Physiol. 45: 245–260, 1983.
Duman, J.G., Olsen, T.M.: Thermal hysteresis protein activity in bacteria, fungi, and phylogenetically diverse plants. — Cryobiology 30: 322–328, 1993.
Ewart, K.V., Lin, Q., Hew, C.L.: Structure, function and evolution of antifreeze proteins. — Cell. mol. Life Sci. 55: 271–283, 1999.
Griffith, M., Ewart, K.: Antifreeze proteins and their potential use in frozen foods. — Biotech. Adv. 13: 375–402, 1995.
Griffith, M., Antikainen, M.: Extracellular ice formation in freezing-tolerant plants. — Adv. Low Temp. Biol. 3: 107–139, 1996.
Griffith, M., Yaish, M.W.F.: Antifreeze proteins in overwintering plants: a tale of two activities. — Trends Plant Sci. 9: 399–405, 2004.
Guerra-Guimaraes, L., Silva, M.C., Struck, C., Loureiro, A., Nicole, M., Rodrigues, C.J., Jr., Ricardo, C.P.P.: Chitinases of Coffea arabica genotypes resistant to orange rust Hemileia vastatrix. — Biol. Plant. 53: 702–706, 2009.
Hansen, T.N., Baust, J.G.: Differential scanning calorimetric analysis of antifreeze protein activity in the common mealworm, Tenebrio molitor. — Biochim. biophys. Acta 957: 217–221, 1988.
Harlow, E., Lane, D. (ed.): Antibodies: A laboratory manual. — Cold Spring Harbor Laboratory Press, Plainview — New York 1988.
Hon, W.C., Griffith, M., Chong, P., Yang, D.S.C.: Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.). — Plant Physiol. 104: 971–980, 1994.
Kobashigawa, Y., Nishimiya, Y., Miura, K., Ohgiyab, S., Miura, A., Tsuda, S.: A part of ice nucleation protein exhibits the ice-binding ability. — FEBS Lett. 579: 1493–1497, 2005.
Kreps, J.A., Wu, Y.J., Chang, H.S., Zhu, T., Wang, X., Harper, J.F.: Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. — Plant Physiol. 130: 2129–2141, 2002.
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. — Nature 277: 680–685, 1970.
Moffat, B., Ewart, V., Eastman, A.: Cold comfort: plant antifreeze proteins. — Physiol. Plant. 126: 5–16, 2006.
Nakamura, T., Ishikawa, M., Nakatani, H., Oda, A.: Characterisation of cold-responsive extracellular chitinase in bromegrass cell cultures and its relationship to antifreeze activity. — Plant Physiol. 147: 391–401, 2008.
Nishizawa, Y., Saruta, M., Nakazono, K., Nishio, Z., Soma, M., Yoshida, T.: Characterization of transgenic rice plants over-expressing the stress-inducible β-glucanase gene Gns1. — Plant mol. Biol. 51: 143–152, 2003.
Patil, R.S., Ghormade, V., Deshpande, M.V.: Chitinolytic enzymes: an exploration. — Enzyme Microbiol. Tech. 26: 473–483, 2000.
Pihakaski-Maunsbach, K., Moffatt, B., Testillano, P., Risueno, M., Yeh, S.S., Griffith, M., Maunsbach, A.B.: Genes encoding chitinase-antifreeze proteins are regulated by cold and expressed by all cell types in winter rye shoots. — Physiol. Plant. 112: 359–371, 2001.
Sambrook, J., Fritsch, E.F., Maniatis, T. (ed.): Molecular Cloning: A Laboratory Manual. 2nd Ed. — Cold Spring Harbour Laboratory Press, Plainview — New York 1989.
Sidebottom, C., Buckley, S., Pudney, P., Twigg, S., Jarman, C., Holt, C.: Heat-stable antifreeze protein from grass. — Nature. 406: 256, 2000.
Thomashow, M.F.: Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. — Annu. Rev. Plant Physiol. Plant mol. Biol. 50: 571–599, 1999.
Tronsmo, A., Harman, G.E.: Detection and quantification of N-acetyl-B-D-glucosaminidase, chitobiosidase, and endochitinase in solutions and on gels. — Anal. Biochem. 208: 74–79, 1993.
Von Heijne, G.: A new method for predicting signal sequence cleavage sites. — Nucl. Acids Res. 14: 4683–4690, 1986.
Wang, H.L., Tao, J.J., He, L.G., Zhao, Y.J., Xu, M., Liu, D.C., Sun, Z.H.: cDBNA cloning and expression analysis of a Poncirus trifoliata DBF gene. — Biol. Plant. 53: 625–630, 2009.
Xin, Z., Browse, J.: Cold comfort farm: the acclimation of plants to freezing temperatures. — Plant Cell Environ. 23: 893–902, 2000.
Xiong, L., Schumaker, K.S., Zhu, J.K.: Cell signaling during cold, drought, and salt stress. — Plant Cell 14(Suppl.): S165–S183, 2002.
Xu, Y., Zhu, Q., Panbangred, W., Shirasu, K., Lamb, C.: Regulation, expression and function of a new basic chitinase gene in rice (Oryza sativa L.). — Plant mol. Biol. 30: 387–401, 1996.
Yeh, S., Moffatt, B., Griffith, M., Xiong, F., Yang, D.S.C., Wiseman, S.B.: Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. — Plant Physiol. 124: 1251–1265, 2000.
Zhang, X.-Y., Liang, C., Wang, G.-P., Luo, Y., Wang, W.: The protection of wheat plasma membrane under cold stress by glycine betaine overproduction. — Biol. Plant. 54: 83–88, 2010.
Acknowledgements
We thank Dr. Y.-C. Xu (Nanjing Agricultural University) for providing the wintersweet seeds and Ms. L.-X. Zhou (Jilin University) for collecting the wintersweet corolla material. We also thank Mr. R. Homfray (University of Wolverhampton) for technical help and advice during the preparation of this manuscript. This work was partially supported by the National Natural Science Foundation of China (No.30871607), the 863 Program (2006BAD08A08), and the Applied Basic Research Programs of Jilin Sci. & Tech.Comm. (No. 20060544).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, S.H., Wei, Y., Liu, J.L. et al. An apoplastic chitinase CpCHT1 isolated from the corolla of wintersweet exhibits both antifreeze and antifungal activities. Biol Plant 55, 141–148 (2011). https://doi.org/10.1007/s10535-011-0019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-011-0019-5