Abstract
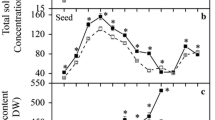
Changes in the activities of sucrose synthase (SuSy), ADP-glucose pyrophosphorylase (AGPase), UDP-glucose pyrophosphorylase (UGPase), alkaline inorganic pyrophosphatase, 3-phosphoglycerate (3-PGA) phosphatase and amylases were monitored in relation to accumulation of starch in developing pods of mung bean (Vigna radiata L.). With the advancement in the seed development, the contents of starch rose with a concomitant fall in the branch of inflorescence and podwall after 10 d after flowering. The activity of UDPase in all the three pod tissues remained higher than the activity of AGPase showing it to be an important enzyme controlling carbon flux. The activity of alkaline inorganic pyrophosphatase in developing seed in contrast to 3-PGA phosphatase correlated with starch accumulation rate. Activity of β-amylase increased in all the pod tissues till maturity. It appears that the cooperative action of SuSy, UGPase and AGPase controls the efficient partitioning of sucrose into ADP glucose and thereby regulate the seed sink strength of the mung bean.
Similar content being viewed by others
Abbreviations
- ADPGlc:
-
ADP-glucose
- AGPase:
-
ADP-glucose pyrophosphorylase
- BI:
-
branch of inflorescence
- DAF:
-
days after flowering
- G1P:
-
glucose-1-phosphate
- 3-PGA:
-
3-phosphoglycerate
- Pi:
-
inorganic phosphate
- PW:
-
podwall
- SuSy:
-
sucrose synthase
- UGPase:
-
UDP-glucose pyrophosphorylase
- UDPGlc:
-
UDP-glucose
References
Bhatia, S., Singh, R.: Calcium-mediated conversion of sucrose to starch in relation to the activities of amylases and sucrose-metabolizing enzymes in sorghum grains raised through liquid culture.-Indian J. Biochem. Biophys. 37: 135–139, 2000.
Bhullar, S.S., Singh, R., Sital, J.S., Bhatia, I.S.: Conversion of sucrose to starch in developing Pennisetum typhoides grain.-Physiol. Plant. 49: 248–254, 1985.
Chopra, J., Kaur, N., Gupta, A.K.: Ontogenic changes in enzymes of carbon metabolism in relation to carbohydrate status in developing mung bean reproductive structures.-Phytochemistry 53: 539–548, 2000.
Chopra, J., Kaur, N., Gupta, A.K.: Changes in the activities of carbon metabolizing enzymes with pod development in lentil (Lens culinaris L.).-Acta Physiol. Plant. 25: 185–191, 2003.
Denyer, K., Dunlap, F., Thorbjornsen, T., Keeling, P., Smith, A.M.: The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial.-Plant Physiol. 112: 779–785, 1996.
Doan, D.N.P., Rudi, H., Olsen, O.-A.: The allosterically unregulated isoform of ADP glucose pyrophosphorylase from barley endosperm is most likely source of ADP glucose incorporated into endosperm starch.-Plant Physiol. 112: 965–975, 1999.
Doehlert, D.C., Duke, S.H.: Specific determination of α-amylase activity in crude plant extracts containing β-amylase.-Plant Physiol. 71: 229–234, 1983.
Doehlert, D.C., Kuo, T.M.: Sugar metabolism in developing kernels of starch-deficient endosperm mutants of maize.-Plant Physiol. 92: 990–994, 1990.
Duffus, C., Rosie, R.: Starch hydrolysing enzymes in the developing barley grain.-Planta 109: 153–160, 1973.
Giese, H., Hejgaard, J.: Synthesis of salt-soluble proteins in barley. Pulse-labeling study of grain filling in liquid-cultured detached spikes.-Planta 161: 172–177,1984.
Gomez-Casati, D.F., Iglesias, A.A.: ADP-glucose pyrophosphorylase from wheat endosperm. Purification and characterization of an enzyme with novel regulatory properties.-Planta 214: 428–434, 2002.
Gross, P., apRees, T.: Alkaline inorganic pyrophosphatase and starch synthesis in amyloplasts.-Planta 167: 140–145, 1986.
Hara-Nishimura, I., Nishimura, M., Daussant, J.: Conversion of free β-amylase to bound β-amylase on starch granules in the barley endosperm during desiccation phase of seed development.-Protoplasma 134: 149–153, 1986.
Hylton, C., Smith, A.M.: The rb mutation of peas causes structural and regulatory changes in ADP-Glc phosphorylase from developing embryos.-Plant Physiol. 99: 1626–1634, 1992.
Kaur, S., Gupta, A.K., Kaur, N.: Effect of kinetin on starch and sucrose metabolising enzymes in salt stressed chickpea seedlings.-Biol. Plant. 46: 67–72, 2003.
Kleczkowski, L.A.: Glucose activation and metabolism through UDP-glucose pyrophosphorylase in plants.-Phytochemistry 37: 1507–1515, 1994.
Kleczkowski, L.A.: Back to the drawing board: redefining starch synthesis in cereals.-Trends Plant Sci. 1: 363–364, 1996.
Kleczkowski, L.A.: A phosphoglycerate to inorganic phosphate ratio is the major factor in controlling starch levels in chloroplasts via ADP-glucose pyrophosphorylase regulation.-FEBS Lett. 448: 153–156, 1999.
Kumar, R., Singh, R.: Alkaline inorganic pyrophosphatase from immature wheat grains.-Phytochemistry 22: 2405–2407, 1983.
Lowry, O.H., Rosebrough, N.J., Farr, A.R., Randall, R.J.: Protein measurement with Folin phenol reagent.-J. biol. Chem. 193: 265–275, 1951.
Minamikawa, T., Yamauchi, D., Wada, S., Takeuchi, H.: Expression of α-amylase in Phaseolus vulgaris and Vigna mungo plants.-Plant Cell Physiol. 33: 253–258, 1992.
Muller-Rober, B., Kossman, J.: Approaches to influence starch quantity and starch quality in transgenic plants.-Plant Cell Environ. 17: 601–613, 1994.
Muller-Rober, B.T., Kossmann, J., Hannah, L.C., Willmitzer, L., Sonnewald, U.: One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose.-Mol. gen. Genet. 224: 136–146, 1990.
Pathre, U.V., Sinha, A.K., Shirke, P.A., Rande, S.A.: Diurnal and seasonal modulation of sucrose phosphate synthase activity in leaves of Prosopis juliflora.-Biol. Plant. 48: 227–235, 2004.
Peoples, M.B., Atkins, C.A., Pate, J.S., Murray, D.R.: Nitrogen nutrition and metabolic interconversions of nitrogenous solutes in developing cowpea fruit.-Plant Physiol. 77: 382–388, 1985.
Pozueta-Romero, J., Perata, P., Akazawa, T.: Sucrose-starch conversion in heterotrophic tissues of plants.-Crit. Rev. Plant Sci. 18: 489–525, 1999.
Ren, H., Madison, J.T., Thompson, J.F.: Identification of an ethanol-soluble protein as β-amylase and its purification from soybean seeds.-Phytochemistry 33: 535–539, 1993a.
Ren, H., Thompson, J.F., Madison, J.T.: Biochemical and physiological studies of soybean β-amylase.-Phytochemistry 33: 541–545, 1993b.
Rinderknecht, H., Wilding, P., Haverback, B.J.: A new method for determination of α-amylase.-Experentia 23: 805–805. 1967
Robertson, D., Beech, I., Bolwell, G.P.: Regulation of the enzymes of UDP-sugar metabolism during differentiation of french bean.-Phytochemistry 39: 21–28, 1995.
Rudi, H., Doan, D.N.P., Olsen, O.-A.: A (His)6-tagged recombinant barley (Hordeum vulgare L.) endosperm ADPglucose pyrophosphorylase expressed in the baculovirus-insect cell system is insensitive to allosteric regulation by 3-phosphoglycerate and inorganic phosphate.-FEBS Lett. 419: 124–130, 1997.
Sharma, N., Kaur, N., Gupta, A.K.: Effect of chlorocholine chloride sprays on carbohydrate composition and activities of sucrose metabolizing enzymes in potato (Solanum tuberosum L.).-Plant Growth Regul. 26: 97–103, 1998.
Singh, R., Asthir, B.: Free sugars in relation to starch accumulation in developing sorghum caryopsis.-Physiol. Plant. 74: 58–65, 1988.
Sivak, M.N., Preiss, J.: Advances in Food and Nutrition Research. Starch: Basic Science to Biotechnology.-Academic Press, San Diego 1998.
Thorbjornsen, T., Villand, P., Denyer, K., Olsen, O.-A., Smith, A.M.: Distinct isoforms of ADP glucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm.-Plant J. 10: 243–250, 1996.
Turner, J.F.: Starch synthesis and changes in uridine diphosphate glucose pyrophosphorylase and adenosine diphosphate glucose pyrophosphorylase in the developing wheat grain.-Aust. J. biol. Sci. 22: 1321–1327, 1969.
Villareal, R.M., Juliano, B.O.: Some properties of 3-phosphoglycerate phosphatase from developing rice grain.-Plant Physiol. 59: 134–138, 1977.
Weber, H., Heim, U., Borisjuk, L., Wobus, U.: Cell-type specific, coordinate expression of two ADP glucose pyrophosphorylase genes in relation to starch biosynthesis during seed development in Vicia faba L.-Planta 195: 352–361, 1995.
Ziegler, P.: Cereal beta-amylases.-J. Cereal Sci. 29: 195–204, 1999.
Zrenner, R., Salanoubat, M., Willmitzer, L., Sonnewald, U.: Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.).-Plant J. 7: 97–107, 1995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chopra, J., Kaur, N. & Gupta, A.K. Role of enzymes of sucrose-starch conversion in seed sink strength in mung bean. Biol Plant 49, 561–566 (2005). https://doi.org/10.1007/s10535-005-0050-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10535-005-0050-5