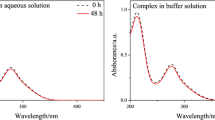
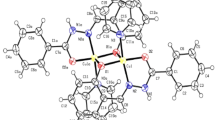
Abstract
In the present work the two new Cu(II) complexes, (µ-acetato)-bis(2,2′-bipyridine)-copper [Cu(bpy)2(CH3CO2)] and bromidotetrakis(2-methyl-1H-imidazole)-copper bromide [Cu(2-methylimid)4Br]Br have been synthesized by liquid assisted mechanochemical method. The [Cu(bpy)2(CH3CO2)] complex (1) and [Cu(2-methylimid)4Br]Br complex (2) characterised by IR and UV–visible spectroscopy and the structure are confirmed by XRD diffraction studies. Complex (1) crystallized in the Monoclinic with the space group of C2/c where a = 24.312(5) Å, b = 8.5892(18) Å, c = 14.559(3) Å, α = 90°, β = 106.177(7)° and γ = 90° and Complex (2) crystallized in the Tetragonal with the space group of P4nc, a = 9.9259(2) Å, b = 9.9259(2) Å, c = 10.9357(2) Å, α = 90°, β = 90° and γ = 90°. The complex (1) has distorted octahedral geometry where the acetate ligand showed bidentate bridging with the central metal ion and complex (2) has slightly deformed square pyramidal geometry. The HOMO–LUMO energy gap value and the low chemical potential showed that the complex (2) is stable and difficult to polarize compare to complex (1). The molecular docking study of complexes with the HIV instasome nucleoprotein showed the binding energy values − 7.1 and − 5.3 kcal/mol for complex (1) and complex (2) respectively. The negative binding energy values showed the complexes have affinity to bind with HIV instasome nucleoproteins. The in-silico pharmacokinetic study of the complex (1) and complex (2) showed non AMES toxicity, non-carcinogens and low honey Bee toxicity but weakly inhibit Human Ether—a-go-go-related gene.
Graphical Abstract















Similar content being viewed by others
References
Abumelha HM, Alkhatib F, Alzahrani S, Abualnaja M, Alsaigh S, Alfaifi MY, Althagafi I, El-Metwaly N (2021) Synthesis and characterization for pharmaceutical models from Co(II), Ni(II) and Cu(II)-thiophene complexes; apoptosis, various theoretical studies and pharmacophore modelling. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.115483
Adams CJ, Haddow MF, Hughes RJI, Kurawa MA, Orpen AG (2010) Coordination chemistry of platinum and palladium in the solid-state: synthesis of imidazole and pyrazole complexes. Dalton Trans 39:3714. https://doi.org/10.1039/b919665j
Alam MJ, Ahmad S (2014) Molecular structure, anharmonic vibrational analysis and electronic spectra of o-, m-, p-iodonitrobenzene using DFT calculations. J Mol Struct 1059:239–254. https://doi.org/10.1016/j.molstruc.2013.12.002
Andrade GR, Kunsminskas J, Pizzuti L, dos Anjos A, Inglez SD, Tirloni B, Suegama PH (2015) Synthesis and X-ray structural characterization of square-pyramidal copper(II) complex with aminoguanidine derivative, Inorganic Chem. Com 61:210–213. https://doi.org/10.1016/j.inoche.2015.09.022
Anoop MR, Binil PS, Suma S, Sudarsanakumar MR, Sheena Mary Y, Varghese HT, Yohannan Panicker C (2010) Vibrational spectroscopic studies and computational study of ethyl methyl ketone thiosemicarbazone. J Mol Struct 969:48–54. https://doi.org/10.1016/j.molstruc.2010.01.041
Arunadevi A, Raman N (2020) Indole-derived water-soluble N, O bi-dentate ligand-based mononuclear transition metal complexes: in silico and in vitro biological screening, molecular docking and macromolecule interaction studies. J Biomol Struct Dyn 38(5):1499–1513. https://doi.org/10.1080/07391102.2019.1611475
Bacchi A, Biemmi M, Carcelli M, Carta F, Compari C, Fisicaro E, Rogolino D, Sechi M, Sippel M, Sotriffer CA, Sanchez TW, Neamati N (2008) From ligand to complexes: part 2: remarks on human immunodeficiency virus type 1 integrase inhibition by β-diketo acid metal complexes. J Med Chem 51(22):7253–7264. https://doi.org/10.1021/jm800893q
Bacchi A, Carcelli M, Compari C, Fisicaro E, Pala N, Rispoli G, Rogolino D, Sanchez TW, Sechi M, Neamati N (2011) HIV-1 IN strand transfer chelating inhibitors: a focus on metal binding. Mol Pharma 8(2):507–519. https://doi.org/10.1021/mp100343x
Balaiah B, Sastry BA, Chary MN, Ponticelli G, Massacesi M (1982) IR, EPR and optical absorption studies of some 2,2′-bipyridine complexes of Copper(II). J Mol Struct 78(3):289–297. https://doi.org/10.1016/0022-2860(82)80015-X
Belkhir-Talbi D, Makhloufi-Chebli M, Terrachet-Bouaziz S, Hikem-Oukacha D, Ghemmit N, Ismaili L, Silva AMS, Hamdi M (2019) Synthesis, characterization, theoretical studies, ADMET and drug-Likeness analysis: electrochemical and biological activities of metal complexes of 3-(2-hydroxybenzoyl)-2H-chromen-2-one. J Mol Struct 1179:495–505. https://doi.org/10.1016/j.molstruc.2018.11.035
Bianco MD, Marinho DI, Hoelz LV, Bastos MM, Boechat N (2021) Pyrroles as privileged scaffolds in the search for new potential HIV inhibitors. Pharmaceuticals. https://doi.org/10.3390/ph14090893
Bill E, Bothe E, Chaudhuri P, Chlopek K, Herebian D, Kokatam S, Ray K, Weyhermüller T, Neese F, Wieghardt K (2005) Molecular and electronic structure of four- and five-coordinate cobalt complexes containing two o-phenylenediamine- or two o-aminophenol-type ligands at various oxidation levels: an experimental, density functional, and correlated ab initio Study. Chem Eur J 11(1):204–224. https://doi.org/10.1002/chem.200400850
Biovia DS (2015) Discovery studio modeling environment. Dassault System Release, San Diego, CA, USA
Boos DD, Stefanski LA (2011) P-value precision and reproducibility. Am Stat 65(4):213–221. https://doi.org/10.1198/tas.2011.10129
Boudjedir A, Kraim K, Saihi Y et al (2021) A computational molecular docking study of camptothecin similars as inhibitors for topoisomerase 1. Struct Chem 32:689–697. https://doi.org/10.1007/s11224-020-01633-6
Bowmaker GA (2013) Solvent-assisted mechanochemistry. Chem Comm 49(4):334–348. https://doi.org/10.1039/C2CC35694E
Brünger AT (1992) Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355(6359):472–475. https://doi.org/10.1038/355472a0
Carcelli M, Bacchi A, Pelagatti P, Rispoli G, Rogolino D, Sanchez TW, Sechi M, Neamati N (2013) Ruthenium arene complexes as HIV-1 integrase strand transfer inhibitors. Spec Issue Bioinorg Chem HNO 118:74–82. https://doi.org/10.1016/j.jinorgbio.2012.09.021
Carcelli M, Rogolino D, Sechi M, Rispoli G, Fisicaro E, Compari C, Grandi N, Corona A, Tramontano E, Pannecouque C, Naesens L (2014) Antiretroviral activity of metal-chelating HIV-1 integrase inhibitors. Eur J Med Chem 83:594–600. https://doi.org/10.1016/j.ejmech.2014.06.055
Castellucci E, Angeloni L, Neto N, Sbrana G (1979) IR and Raman spectra of A 2,2′-bipyridine single crystal: internal modes. Chem Phys 43(3):365–373. https://doi.org/10.1016/0301-0104(79)85204-0
Ćendić M, Matović ZD, Deeth RJ (2013) Molecular modeling for Cu(II)-aminopolycarboxylate complexes: structures, conformational energies, and ligand binding affinities. J Comput Chem 34:2687–2696. https://doi.org/10.1002/jcc.23437
Cinčić D, Kaitner B (2011) Schiff base derived from 2-hydroxy-1-naphthaldehyde and liquid-assisted mechanochemical synthesis of its isostructural Cu(II) and Co(II) complexes. Cryst Eng Comm 13(13):4351–4357. https://doi.org/10.1039/C0CE00421A
Colthup NB, Daly LH, Wiberly SE (1990) Introduction to infrared and Raman Spectroscopy, 3rd edn. Academic Press, Boston
Cook NJ, Li W, Berta D, Badaoui M, Ballandras-Colas A, Nans A, Kotecha A, Rosta E, Engelman AN, Cherepanov P (2020) Structural basis of second-generation HIV integrase inhibitor action and viral resistance. Science 367:806–810
Costamagna J, Caruso F, Vargas J, Manriquez V (1998) Planar or tetrahedral coordination in copper(II) complexes induced by bromo substitution. Inorg Chim Acta 267(1):151–158. https://doi.org/10.1016/S0020-1693(97)05571-0
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Dimić DS, Kaluđerović GN, Avdović EH, Milenković DA, Živanović MN, Potočňák I, Samoľová E, Dimitrijević MS, Saso L, Marković ZS, Dimitrić Marković JM (2022) Synthesis, crystallographic, quantum chemical, antitumor, and molecular docking/dynamic studies of 4-hydroxycoumarin-neurotransmitter derivatives. Int J Mol Sci. https://doi.org/10.3390/ijms23021001
Dutta B, Das D, Datta J, Chandra A, Jana S, Sinha C, Ray PP, Mir MH (2019) Synthesis of a Zn(II)-based 1D zigzag coordination polymer for the fabrication of optoelectronic devices with remarkably high photosensitivity. Inorg Chem Front 6(5):1245–1252. https://doi.org/10.1039/C9QI00162J
Eichhorn T, Kolbe F, Mišić S, Dimić D, Morgan I, Saoud M, Milenković D, Marković Z, Rüffer T, Dimitrić Marković J, Kaluđerović GN (2022) Synthesis, crystallographic structure, theoretical analysis, molecular docking studies, and biological activity evaluation of binuclear Ru (II)-1-naphthylhydrazine complex. Int J Mol Sci 24(1):689. https://doi.org/10.3390/ijms24010689
Ekowo LC, Eze SI, Ezeorah JC, Groutso T, Atiga S, Lane JR, Okafor S, Akpomie KG, Okparaeke OC (2020) Synthesis, structure, Hirshfeld surface, DFT and in silico studies of 4-[(E)-(2, 5-dimethoxybenzylidene) amino]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-3H-pyrazol-3-one (DMAP) and its metal complexes. J Mol Struct 1210:127994. https://doi.org/10.1016/j.molstruc.2020.127994
Farghaly TA, El-Metwaly N, Al-Soliemy AM, Katouah HA, Muhammad ZA, Sabour R (2021) Synthesis, molecular docking and antitumor activity of new dithiazoles. Polycycl Aromat Compd 41(8):1591–1607. https://doi.org/10.1080/10406638.2019.1689512
Farrugia LJ (1997) ORTEP-3 for Windows—a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Cryst 30:565. https://doi.org/10.1107/S0021889897003117
Farrugia LJ (1999) WinGX suite for small-molecule single-crystal crystallography. J Appl Cryst 32:837–838. https://doi.org/10.1107/S0021889899006020
Filippou V, Blickle S, Bubrin M, Kaim W (2021) Intramolecular charge transfer in ruthenium complexes [Ru(acac)2(ciq)] with ambidentate camphoriminoquinone (ciq) ligands. Anorg Allg Chem 2021(647):525
Fonteh PN, Keter FK, Meyer D (2011) New bis(thiosemicarbazonate) gold(III) complexes inhibit HIV replication at cytostatic concentrations: potential for incorporation into virostatic cocktails. J Inorg Biochem 105(9):1173–1180. https://doi.org/10.1016/j.jinorgbio.2011.05.011
Galini M, Salehi M, Kubicki M, Bayat M, Malekshah RE (2020) Synthesis, structural characterization, DFT and molecular simulation study of new zinc-Schiff base complex and its application as a precursor for preparation of ZnO nanoparticle. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.127715
Godlewska S, Jezierska J, Baranowska K, Augustin E, Dołęga A (2013) Copper(II) complexes with substituted imidazole and chlorido ligands: X-ray, UV–Vis, magnetic and EPR studies and chemotherapeutic potential. Polyhedron 65:288–297. https://doi.org/10.1016/j.poly.2013.08.039
Graddon DP, Mockler GM (1968) Copper(II) complexes of o-hydroxyarylcarbonyl compounds. Aust J Chem 21:617–629
Grawenhoff J, Engelman AN (2017) Retroviral integrase protein and intasome nucleoprotein complex structures. World J Biol Chem 8(1):32–44. https://doi.org/10.4331/wjbc.v8.i1.32
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723. https://doi.org/10.1002/elps.1150181505
Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H (1999) Inhibition of HIV-1 infection by zinc group metal compounds. Antivir Res 43(2):123–133. https://doi.org/10.1016/S0166-3542(99)00040-6
Hathaway BJ, Procter IM, Slade RC, Tomlinson AAG (1969) The electronic properties and stereochemistry of the copper (II) ion. Part VI. Bis(bipyridyl)copper(II) complexes. J Chem Soc A. https://doi.org/10.1039/J19690002219
Hathaway BJ, Ray N, Kennedy D, O’Brien N, Murphy B (1980) The structures of acetatobis(2,2′-bipyridyl)copper(II) perchlorate monohydrate and tetrafluoroborate—cation distortion isomers. Acta Cryst B36:1371–1377. https://doi.org/10.1107/S0567740880006103
Jevtovic V, Alshammari N, Latif S, Alsukaibi AK, Humaidi J, Alanazi TYA, Abdulaziz F, Matalka SI, Pantelić NĐ, Marković M, Rakić A, Dimić D (2022) Synthesis, crystal structure, theoretical calculations, antibacterial activity, electrochemical behavior, and molecular docking of Ni(II) and Cu(II) complexes with pyridoxal-semicarbazone. Molecules. https://doi.org/10.3390/molecules27196322
Jeyaraman P, Alagarraj A, Natarajan R (2020) In silico and in vitro studies of transition metal complexes derived from curcumin–isoniazid Schiff base. J Biomol Struct Dyn 38(2):488–499. https://doi.org/10.1080/07391102.2019.1581090
Jeyaraman P, Samuel M, Johnson A, Raman N (2021) Synthesis, characterization, ADMET, in vitro and in vivo studies of mixed ligand metal complexes from a curcumin Schiff base and lawsone. Nucleosides Nucleotides Nucleic Acids 40(3):242–263. https://doi.org/10.1080/15257770.2020.1867865
Jianmin L, Jianbin Z, Yanxiong K, Xintao W (1996) A novel structure of bipyridine coordinated with Copper (II): [Cu(bipy) (H2O)3] · (NO3)2. Cryst Res Technol 31:589–593. https://doi.org/10.1002/crat.2170310509
Jin S, Wang D, Xu Y (2012) Five new metal(II) complexes with 3-D network structures based on carboxylate and bis(imidazole) ligands: syntheses and structures. J Coord Chem 65(11):1953–1969. https://doi.org/10.1080/00958972.2012.688116
Jóźwik IK, Passos DO, Lyumkis D (2020) Structural biology of HIV integrase strand transfer inhibitors. Trends Pharmacol Sci 41(9):611–626. https://doi.org/10.1016/j.tips.2020.06.003
Karplus PA, Diederichs K (2012) Linking crystallographic model and data quality. Science 336:1030–1033. https://doi.org/10.1126/science.1218231
Khalil MMH, Mashaly MM (2008) New transition and actinide metal complexes of 2-carboxy-phenylhydrazo-benzoylacetone ligand: synthesis, characterization and biological study. Chin J Chem 26:1669–1677. https://doi.org/10.1002/cjoc.200890302
Khan TA, Ghani SS, Naseem S (2010) Interaction of Co(II), Ni(II), Cu(II), and Zn(II) with 12- and 14-membered macrocycles containing O2N2 donors. J Coord Chem 63(24):4411–4420. https://doi.org/10.1080/00958972.2010.53552
Koo BK (2001) Synthesis, crystal structure, and characterization of Copper(II) acetate complex. Bull Korean Chem Soc 22:113–116
Madi AA, Haffar D, Benghanem F, Ghedjati S, Toukal L, Dorcet V, Bourzami R (2021) Synthesis, crystal structure, electrochemical, theoretical studies and antioxidant activities of new schiff base. J Mol Struct 1227:129368. https://doi.org/10.1016/j.molstruc.2020.129368
McShane BB, Gal D, Gelman A, Robert C, Tackett JL (2019) Abandon statistical significance. Am Stat 73(Suppl 1):235–245. https://doi.org/10.1080/00031305.2018.1527253
Morzyk-Ociepa B, Różycka-Sokołowska E, Michalska D (2012) Revised crystal and molecular structure, FT-IR spectra and DFT studies of chlorotetrakis(imidazole)copper(II) chloride. J Mol Struct 1028:49–56. https://doi.org/10.1016/j.molstruc.2012.06.028
Nareetsile F, Matshwele JTP, Ndlovu S, Ngaski M (2020) Transition metal complexes with HIV/AIDS inhibitory properties. Chem Sci Rev Lett 3(3):140–160
Neese F (2012) The ORCA program system. WIRES Comput Mol Sci 2(1):73–78. https://doi.org/10.1002/wcms.81
Palmer RA, Piper TS (1966) 2,2′-Bipyridine complexes. I. Polarized crystal spectra of Tris (2,2′-bipyridine)copper(II), -nickel(II), -cobalt(II), -iron(II), and -ruthenium(II). Inorg Chem 5(5):864–878. https://doi.org/10.1021/ic50039a034
Passos DO, Li M, Jóźwik IK, Zhao XZ, Santos-Martins D, Yang R, Smith SJ, Jeon Y, Forli S, Hughes SH, Burke TR Jr, Craigie R, Lyumkis D (2020) Structural basis for strand-transfer inhibitor binding to HIV intasomes. Science 367(6479):810–814. https://doi.org/10.1126/science.aay8015
Patel DK, Martín AD, Lazarte DC, Pérez JMG, Gutiérrez JN (2022) Synthesis, characterization, and quantum chemical study of cobalt(II) chelates with N-phenethyl-iminodiacetate(2-)-like ligands. Influence of p-(R)-phenethyl group on crystal pattern. J Coord Chem 75:2814–2828. https://doi.org/10.1080/00958972.2022.2140407
Pelosi G, Bisceglie F, Bignami F, Ronzi P, Schiavone P, Re MC, Casoli C, Pilotti E (2010) Antiretroviral activity of thiosemicarbazone metal complexes. J Med Chem 53(24):8765–8769. https://doi.org/10.1021/jm1007616
Pisanò G, Cazin CS (2020) Mechanochemical synthesis of Cu(i)-N-heterocyclic carbene complexes. J Green Chem 22:5253–5256. https://doi.org/10.1039/D0GC01923B
Pommier Y, Johnson AA, Marchand C (2005) Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 4(3):236–248
Putterill B, Rono C, Makhubela B, Meyer D, Gama N (2022) Triazolyl Ru(II), Os(II), and Ir(III) complexes as potential HIV-1 inhibitors. Biometals 35(4):771–784. https://doi.org/10.1007/s10534-022-00400-w
Radha VP, Chitra S, Jonekirubavathi S, Chung I-M, Kim S-H, Prabakaran M (2020) Transition metal complexes of novel binuclear Schiff base derived from 3,3′-diaminobenzidine: synthesis, characterization, thermal behavior, DFT, antimicrobial and molecular docking studies. J Coord Chem 73(6):1009–1027. https://doi.org/10.1080/00958972.2020.1752372
Rastegarnia S, Pordel M, Allameh S (2019) Synthesis, characterization, and DFT calculations of new fluorescent Cu(II) complexes of heterocyclic ligands. J Struct Chem 60(2):232–240. https://doi.org/10.1134/S0022476619020082
Rathod RV, Mondal D, Bera S (2020) Mechanochemical synthesis of fluorescein-based receptor for CN− ion detection in aqueous solution and cigarette smoke residue. Anal Bioanal Chem 412(13):3177–3186. https://doi.org/10.1007/s00216-020-02573-0
Rogolino D, Carcelli M, Compari C, Luca LD, Ferro S, Fisicaro E, Rispoli G, Neamati N, Debyser Z, Christ F, Chimirri A (2014) Diketoacid chelating ligands as dual inhibitors of HIV-1 integration process. Eur J Med Chem 78:425–430. https://doi.org/10.1016/j.ejmech.2014.03.070
Roy S, Mitra P, Patra AK (2011) Cu(II) complexes with square pyramidal (N2S)CuCl2 chromophore: Jahn–Teller distortion and subsequent effect on spectral and structural properties. Inorg Chim Acta 370:247–253. https://doi.org/10.1016/j.ica.2011.01.068
Sanna D, Sciortino G, Ugone V, Micera G, Garribba E (2016) Nonoxido V(IV) complexes: prediction of the EPR spectrum and electronic structure of simple coordination compounds and Amavadin. Inorg Chem 55(15):7373–7387
Satheesh Chandran PR, Soumya Mol US, Drisya R, Sudarsanakumar MR, Prathapachandra Kurup MR (2017) Structural studies of poly[(μ2-acetato)(μ3-5-aminoisophthalato)diaquacerium(III) monohydrate]: a new three dimensional fluorescent metal-organic framework constructed from dimers of CeO9 polyhedra with hydrophilic ‘S’ shaped channels. J Mol Struct 1137:396–402. https://doi.org/10.1016/j.molstruc.2017.02.024
Savarino A (2006) A historical sketch of the discovery and development of HIV-1 integrase inhibitors. Expert Opin Investig Drugs 15(12):1507–1522
Schlesinger M, Schulze S, Hietschold M, Mehring M (2010) Evaluation of synthetic methods for microporous metal–organic frameworks exemplified by the competitive formation of [Cu2(btc)3(H2O)3] and [Cu2(btc)(OH)(H2O)]. Microp Mesop Mater 132:121–127. https://doi.org/10.1016/j.micromeso.2010.02.008
Sechi M, Bacchi A, Carcelli M, Compari C, Duce E, Fisicaro E, Rogolino D, Gates P, Derudas M, Al-Mawsawi LQ, Neamati N (2006) From ligand to complexes: inhibition of human immunodeficiency virus type 1 integrase by β-Diketo acid metal complexes. J Med Chem 49(14):4248–4426. https://doi.org/10.1021/jm060193m
Seferoğlu N, Toprakçıoğlu G (2019) Detailed theoretical characterization of azo chromophores containing dicyanomethylene acceptor and various coupling components by DFT. J Mol Struct 1181:360–372. https://doi.org/10.1016/j.molstruc.2018.12.080
Shahabadi N, Abbasi AR, Moshtkob A, Shiri F (2019) DNA-binding studies of a new Cu(II) complex containing reverse transcriptase inhibitor and anti-HIV drug zalcitabine. J Coord Chem 72(12):1957–1972. https://doi.org/10.1080/00958972.2019.1620216
Shakeri A, Panahi Y, Johnston TP, Sahebkar A (2019) Biological properties of metal complexes of curcumin. BioFactors 45(3):304–317. https://doi.org/10.1002/biof.1504
Shanty AA, Raghu KG, Mohanan PV (2019) Synthesis, characterization: spectral and theoretical, molecular docking and in vitro studies of copper complexes with HIV RT enzyme. J Mol Struct 1197:154–163. https://doi.org/10.1016/j.molstruc.2019.06.097
Shawan MMAK, Halder SK, Hasan MA (2021) Luteolin and abyssinone II as potential inhibitors of SARS-CoV-2: an in silico molecular modeling approach in battling the COVID-19 outbreak. Bull Natl Res Cent 45:27. https://doi.org/10.1186/s42269-020-00479-6
Sheldrick GM, Schneider TR (1997) SHELXL: High-resolution refinement. Meth Enzymol 277:319–343. https://doi.org/10.1016/S0076-6879(97)77018-6
Sienkiewicz-Gromiuk J (2018) DFT approach to (benzylthio)acetic acid: conformational search, molecular (monomer and dimer) structure, vibrational spectroscopy and some electronic properties. Spectrochim Acta A Mol Biomol Spectrosc 189:116–128. https://doi.org/10.1016/j.saa.2017.07.054
Singh VK, Chamberlain-Clay A, Ong HC, León F, Hum G, Par MY, Daley-Dee P, García F (2021) Multigram mechanochemical synthesis of a salophen complex: a comparative analysis. ACS Sustain Chem Eng 9(3):1152–1160. https://doi.org/10.1021/acssuschemeng.0c06374
Sinnecker S, Neese F, Noodleman L, Lubitz W (2004) Calculating the electron paramagnetic resonance parameters of exchange coupled transition metal complexes using broken symmetry density functional theory: application to a MnIII/MnIV model compound. J Am Chem Soc 126(8):2613–2622. https://doi.org/10.1021/ja0390202
Soumya Mol US, Nair RM, Drisya R, Satheesh Chandran PR, Sudarsanakumar MR, Ng SW, Prathapachandra Kurup MR (2016) Growth and characterization studies of a novel luminescent acetate-bridged barium(II) complex: poly[(μ-diacetato)(tetraphthalato)pentabarium(II)]. Main Group Met Chem 39(5–6):157–165. https://doi.org/10.1515/mgmc-2016-0033
Spek AL (2002) Single-crystal structure validation with the program PLATON. J Appl Cryst 36:7–11
Stoychev GL, Auer AA, Neese F (2017) Automatic generation of auxiliary basis sets. J Chem Theory Comput 13(2):554–562. https://doi.org/10.1021/acs.jctc.6b01041
Tanabe K, Saeki S (1975) Computer retrieval of infrared spectra by a correlation coefficient method. Anal Chem 47(1):118–122. https://doi.org/10.1021/ac60351a041
Trott O, Olson AJ (2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Tsiang M, Jones GS, Niedziela-Majka A, Kan E, Lansdon EB, Huang W, Hung M, Samuel D, Novikov N, Xu Y, Mitchell M, Guo H, Babaoglu K, Liu X, Geleziunas R, Sakowicz R (2012) New class of HIV-1 integrase (IN) inhibitors with a dual mode of action. J Biol Chem 287(25):21189–21203
Tsiang M, Jones GS, Goldsmith J, Mulato A, Hansen D, Kan E, Tsai L, Bam RA, Stepan G, Stray KM, Niedziela-Majka A, Yant SR, Yu H, Kukolj G, Cihlar T, Lazerwith SE, White KL, Jin H (2016) Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 60:7086–7097
Üstün E, Çol Ayvaz M, Sönmez Çelebi M, Aşcı G, Demir S, Özdemir İ (2016) Structure, CO-releasing property, electrochemistry, DFT calculation, and antioxidant activity of benzimidazole derivative substituted [Mn(CO)3(bpy)L]PF6 type novel manganese complexes. Inorg Chim Acta 450:182–189. https://doi.org/10.1016/j.ica.2016.05.027
Uzun S, Esen Z, Koç E, Usta NC, Ceylan M (2019) Experimental and density functional theory (MEP, FMO, NLO, Fukui functions) and antibacterial activity studies on 2-amino-4-(4-nitrophenyl)-5,6-dihydrobenzo [h] quinoline-3-carbonitrile. J Mol Struct 1178:450–457. https://doi.org/10.1016/j.molstruc.2018.10.001
Walsh A, Walsh B, Murphy B, Hathaway BJ (1981) The structures of bis(2,2′-bipyridyl)mononitritocopper(II) tetrafluoroborate and bis(2,2′-bipyridyl)mononitritozinc(II) nitrate. Acta Cryst B37:1512–1520. https://doi.org/10.1107/S056774088100647X
Wang JY, Ling H, Yang W, Carigie R (2001) Structure of a two-domain fragment of HIV-1 integrase: implications for domain organization in the intact protein. EMBO J 20(24):7333–7343
Wang Z, Song R, Chen W, Wang J, Wang P, Zhang Z, Zhang X, Wan F (2022) Vibrational spectra and molecular vibrational behaviors of dibenzyl disulfide, dibenzyl sulphide and bibenzyl. Int J Mol Sci 23(4):1958
Warren KD (1976) Ligand field theory of metal sandwich complexes. Second order effects in the magnetic behaviour of dx configurations. J Phys Chem 19:215–220. https://doi.org/10.1016/S0020-1693(00)91098-3
Xin Hu, Zhang Li, Liu L, Liu G, Jia D, Guancheng Xu (2006) Synthesis and structural characterization of three hydrogen-bonding connected supramolecular complexes of nickel, zinc and copper with 1,3-diphenyl-4-(salicylidene hydrazide)-acetyl-pyrazolone-5 and 2,2′-bipyridine. Inorg Chim Acta 359:633–641. https://doi.org/10.1016/j.ica.2005.10.013
Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Li W, Liu G, Tang Y (2018) admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 35:1067–1069. https://doi.org/10.1093/bioinformatics/bty707
Yavari M, Beyramabadi SA, Morsali A, Bozorgmehr MR (2019) Synthesis, experimental and theoretical studies on N,N′-dipyridoxyl(4-chloro-1,2-phenylenediamine) tetradentate ligand and its copper(II) complex. J Struct Chem 60(8):1243–1255. https://doi.org/10.1134/S0022476619080055
Yufanyi DM, Abbo HS, Titinchi SJJ, Neville T (2020) Platinum(II) and Ruthenium(II) complexes in medicine: antimycobacterial and anti-HIV activities. Coord Chem Rev 414:213285. https://doi.org/10.1016/j.ccr.2020.213285
Zhang J, Xu L, Wong WY (2018) Energy materials based on metal Schiff base complexes. Coord Chem Rev 355:180–198. https://doi.org/10.1016/j.ccr.2017.08.007
Zhu H-F, Fan J, Okamura T, Sun W-Y, Ueyama N (2005) Syntheses and structures of Zinc(II), Silver(I), Copper(II), and Cobalt(II) complexes with imidazole-containing ligand: 1-(1-Imidazolyl)-4-(imidazol-1-ylmethyl)benzene. Cryst Growth Des 5(1):289–294. https://doi.org/10.1021/cg0498857
Acknowledgements
The authors are thankful to CDRI, Lucknow, U.P. (India) for providing the spectral facilities for UV–visible and IR and SAIF-IIT Madras for XRD facility.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
S.A. Khan made significant contribution in the conception of work, study design, interpretation and critically reviewing of the article. R. Jaryal made the contribution in execution of work, acquisition of data, analysis and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
Authors declared that the submitted work is original and not have been published elsewhere in any form or language. Data, Tables and Figures quoted in the article for publication are also original and unpublished work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaryal, R., Khan, S.A. Liquid-assisted mechanochemical synthesis, crystallographic, theoretical and molecular docking study on HIV instasome of novel copper complexes: (µ-acetato)-bis(2,2′-bipyridine)-copper and bromidotetrakis(2-methyl-1H-imidazole)-copper bromide. Biometals 36, 975–996 (2023). https://doi.org/10.1007/s10534-023-00498-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-023-00498-6