Abstract
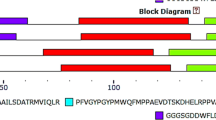
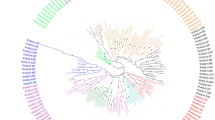
Iron (Fe) is an essential element for plant life. Its deficiency impedes growth and development and excessive iron can cause the toxic effect via the Fenton reaction. Thus, plants have developed various mechanisms to acquire, distribute and utilize Fe for the maintenance of their iron homeostasis at cellular and systemic levels. A basic helix-loop-helix (bHLH) transcription factor family plays essential roles in many regulatory and development processes in plants. In this study, we aimed to understand the roles of bHLH38, bHLH39, bHLH100 and bHLH101 genes for Fe homeostasis in Arabidopsis, tomato, rice, soybean and maize species by using bioinformatics approaches. The gene/protein sequence analyses of these genes demonstrated that all bHLH proteins comprised helix-loop-helix DNA binding domain (PF00010) with varied exon numbers between 2 and 13. The phylogenetic analysis did not reveal a clear distinction between monocot and dicot plants. A total of 61 cis-elements were found in promotor sequences, including biotic and abiotic stress responsiveness, hormone responsiveness, and tissue specific expressions. The some structural divergences were identified in predicted 3D structures of bHLH proteins with different channels numbers. The co-expression network analysis demonstrated that bHLH39 and bHLH101 played more important roles in Fe regulation in Arabidopsis. The digital expression analysis showed various expression profiles of bHLH genes which were identified in developmental stages, anatomical parts, and perturbations. Particularly, bHLH39 and bHLH101 genes were found to be more active genes in Fe homeostasis. As a result, our findings can contribute to understanding of bHLH38, bHLH39, bHLH100 and bHLH101 genes in Fe homeostasis in plants.








Similar content being viewed by others
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaquchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Aoki Y, Okamura Y, Tadaka S, Kinoshita K, Obayashi T (2016) ATTED-II in 2016: a plant coexpression database towards lineage-specific coexpression. Plant Cell Physiol 57:e5
Brumbarova T, Bauer P, Ivanov R (2014) Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci. https://doi.org/10.1016/j.tplants.2014.11.004
Buckhout TJ, Yang TJ, Schmidt W (2009) Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genom 10:147
Celma JR, Pan C, Li W, Lan P, Buckhout TJ, Schmidt W. (2012) The transcriptional response of Arabidopsis leaves to Fe deficiency. Front Plant Sci 4:1–10. Article 276
Colangelo EP, Guennoti ML (2004) The essential basic helix-loop-helix protein fit1 is required for the iron deficiency response. Plant Cell 16:3400–34121
Conorton JM, Balk J, Celma JR (2017) Iron homeostasis in plants—a brief overview. Metallomics 9:813
Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN (2008) Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320(5878):942–945
Eisenhaber F. (2006) Prediction of protein function. In: Discovering biomolecular mechanisms with computational biology. Molecular Biology Intelligence Unit. Springer, Boston
Filiz E, Vatansever R, Ozyigit II (2017) Dissecting a co-expression network of basic helix-loop-helix (bHLH) genes from phosphate (Pi)-starved soybean (Glycine max). Plant Gene 9:19–25
Garcia CMH, Finer JJ (2014) Identification and validation of promoters and cis-acting regulatory elements. Plant Sci 217–218:109–119
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD et al (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana, Louisville, pp 571–607
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:1178–1186
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
He H, Yan J, Yu X, Liang Y, Fang L, Scheller HV, Zhang A (2017) The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem Biophys Res Commun 491(3):834–839. https://doi.org/10.1016/j.bbrc.2017.05.131
Heim MA, Jacoby M, Werber M, Martin C, Weisshaar B, Bailey PC (2003) The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol 20:735–747
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinform. https://doi.org/10.1155/2008/420747
Hudson KA, Hudson ME (2015) A classification of basic helix-loop-helix transcription factors of soybean. Int J Genom. https://doi.org/10.1155/2015/603182
Iyer-Pascuzzi AS, Jackson T, Cui H, Petricka JJ, Busch W, Tsukagoshi H, Benfey PN (2011) Cell identity regulators link development and stress responses in the Arabidopsis root. Dev Cell 21(4):770–782
Jones S (2004) An overview of the basic helix-loop-helix proteins. Genome Biol 5:226
Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10(6):845–858
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS Lett 581:2273–2280
Kim JK, Cho Y, Lee M, Laskowski RA, Ryu SE, Sugihara K, Kim DS (2015) BetaCavityWeb: a webserver for molecular voids and channels. Nucleic Acids Res 43(W1):W413–W418. https://doi.org/10.1093/nar/gkv360
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Laloum T, De Mita S, Gamas P, Baudin M, Niebel A (2013) CCAAT-box binding transcription factors in plants: y so many? Trends Plant Sci 18(3):157–166
Lescot M, De´hais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rombauts S (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327
Li J, Liu B, Cheng F, Wang X, Aarts MGM, Wu J (2014) Expression profiling reveals functionally redundant multiple-copy genes related to zinc, iron and cadmium responses in Brassica Rapa. New Phytol 203:182–194
Liang G, Zhang H, Li X, Ai Q, Yu D (2017) bHLH transcription factor bHLH115 regulates iron homeostasis in Arabidopsis thaliana. J Exp Bot 68(7):1743–1755
Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, Benfey PN (2010) The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22:2219–2236
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Murre C, Bain G, Vandijk MA, Engel I, Furnari BA, Massari ME, Matthews JR, Quong MW, Rivera RR, Stuiver MH (1994) Structure and function of helix-loop-helix proteins. Biochim Biophys Acta 1218:129–135
Nguyen MN, Tan KP, Madhusudhan MS (2011) CLICK -topology-independent comparison of biomolecular 3D structures. Nucleic Acids Res 39:W24–W28
Niu X, Guan Y, Chen S, Li H (2017) Genome-wide analysis of basic helix-loophelix (bHLH) transcription factors in Brachypodium distachyon. BMC Genom 18:619
Pires N, Dolan L (2010) Origin and diversification of basic-helix-loop-helix proteins in plants. Mol Biol 27(4):862–874
Ramamurthy RK, Waters BM (2017) Mapping and characterization of the fefe gene that controls iron uptake in melon (Cucumis melo L.). Front Plant Sci 8:1003
Rasheed S, Bashir K, Matsui A, Tanaka M, Seki M (2016) Transcriptomic analysis of soil-grown Arabidopsis thaliana roots and shoots in response to a drought stress. Front. Plant Sci. 7:180. https://doi.org/10.3389/fpls.2016.00180
Rizhsky L, Liang H, Mittler R (2003) The water-water cycle is essential for chloroplast protection in the absence of stress. J Biol Chem 278(40):38921–38925
Rout GR, Sahoo S (2015) Role of iron in plant growth and metabolism. Rev Agric Sci 3(1–24):2015
Sivitz AB, Hermand V, Curie C, Vert G (2012) Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-Independent Pathway. PLoS ONE 7(9):e44843
Sonnhammer EL, Eddy SR, Durbin R (1997) Pfam: a comprehensive database of protein domain families based on seed alignments. Proteins 28:405–420
Stein RJ, Waters BM (2012) Use of natural variation reveals core genes in the transcriptome of iron-deficient Arabidopsis thaliana roots. J Exp Bot 63(2):1039–1055
Sun H, Fan HJ, Ling HQ (2015) Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genom 16:9
Thomine S, Vert G (2013) Iron transport in plants: better be safe than sorry. Curr Opin Plant Biol 16:322–327
Timothy L, Mikael Bode´n B, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:202–208
Toledo-Ortiz G, Huq E, Quail PH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15:1749–1770
Vatansever R, Filiz E, Ozyigit II (2015) Genome-wide analysis of iron-regulated transporter 1 (irt1) genes in plants. hortic. Environ Biotechnol 56:516–523
Wang H, Klatte M, Jakoby M, Bäumlein H, Weisshaar B, Bauer P (2007) Iron defciency-mediated stress regulation of four subgroup Ib BHLH genes in Arabidopsis thaliana. Planta 226:897–908
Wang N, Cui Y, Liu Y, Fan H, Du J, Huang Z, Yuan Y, Wu H, Ling HQ (2013) Requirement and functional redundancy of Ib Subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol Plant 6(2):503–513
Willard L, Ranjan A, Zhang H, Monzavi H, Boyko RF, Sykes BD, Wishart DS (2003) VADAR: a web server for quantitative evaluation of protein structure quality. Nucleic Acids Res 31(13):3316–3319
Wong DCJ, Gutierrez RL, Gambetta GA, Castellarin SD (2017) Genome-wide analysis of cis-regulatory element structure and discovery of motif-driven gene co-expression networks in grapevine. DNA Res 24(3):311–326
Xiang Q (2015) Molecular genetic aspects of iron and copper cross-talk in Arabidopsis thaliana. Dissertation, University of Nebraska-Lincoln
Xing J, Wang T, Ni Z (2015) Epigenetic regulation of iron homeostasis in Arabidopsis. Plant Signal Behav 10:12
Yousefi M, Nematzadeh GA, Askari H, Nasiri N (2012) Bioinformatics analysis of promoters cis elements and study on genes co-expression of oxidative defense pathway in Oryza sativa L. Plant. Int J Agric Crop Sci 4(17):1240–1245
Yu CS, Chen YC, Lu CH, Hwang JK (2006) Prediction of protein subcellular localization. Proteins 64:643–651
Zhang J, Liu B, Mengshu L, Feng D, Jin H, Wang P, Liu J, Xiong F, Wang J, Wang HB (2015a) The bHLH transcription factor bHLH104 interacts with IAA-leucine resistant3 and modulates iron homeostasis in Arabidopsis. Plant Cell 27:787–805
Zhang X, Luo H, Xu Z, Zhu Y, Ji A, Song J, Chen S (2015b) Genome-wide characterization and analysis of bHLH transcription factors related to tanshinone biosynthesis in Salvia miltiorrhiza. Sci Rep 5:1124
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurt, F., Filiz, E. Genome-wide and comparative analysis of bHLH38, bHLH39, bHLH100 and bHLH101 genes in Arabidopsis, tomato, rice, soybean and maize: insights into iron (Fe) homeostasis. Biometals 31, 489–504 (2018). https://doi.org/10.1007/s10534-018-0095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-018-0095-5