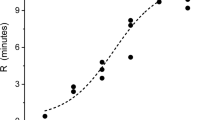
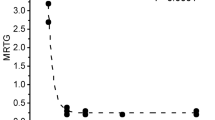
Abstract
Envenomation by hemotoxic enzymes continues to be a major cause of morbidity and mortality throughout the world. With regard to treatment, the gold standard to abrogate coagulopathy caused by these venoms is still the administration of antivenom; however, despite antivenom therapy, coagulopathy still occurs and recurs. Of interest, this laboratory has demonstrated in vitro and in vivo that coagulopathy inducing venom derived from snakes of the family Viperidae exposed to carbon monoxide (CO) is inhibited, potentially by an attached heme. The present investigation sought to determine if venoms derived from snakes of the Elapidae family (taipans and cobras) could also be inhibited with CO or with the metheme inducing agent, O-phenylhydroxylamine (PHA). Assessing changes in coagulation kinetics of human plasma with thrombelastography, venoms from Elapidae snakes were exposed in isolation to CO (five species) or PHA (one specie) and placed in human plasma to assess changes in procoagulant or anticoagulant activity. The procoagulant activity of two taipan venoms and anticoagulant activity of three cobra venoms were significantly inhibited by CO. The venom of the inland taipan was also inhibited by PHA. In sum, these data demonstrate indirectly that the biometal heme is likely bound to these disparate venoms as an intermediary modulatory molecule. In conclusion, CO may not just be a potential therapeutic agent to treat envenomation but also may be a potential modulator of heme as a protective mechanism for venomous snakes against injury from their own proteolytic venoms.






Similar content being viewed by others
References
Berling I, Isbister GK (2015) Hematologic effects and complications of snake envenoming. Transfus Med Rev 29:82–89
Bush SP, Ruha AM, Seifert SA, Morgan DL, Lewis BJ, Arnold TC, Clark RF, Meggs WJ, Toschlog EA, Borron SW, Figge GR, Sollee DR, Shirazi FM, Wolk R, de Chazal I, Quan D, García-Ubbelohde W, Alagón A, Gerkin RD, Boyer LV (2015) Comparison of F(ab’)2 versus Fab antivenom for pit viper envenomation: a prospective, blinded, multicenter, randomized clinical trial. Clin Toxicol 53:37–45
Chester A, Crawford GPM (1982) In vitro coagulant properties of venoms from Australian snakes. Toxicon 20:501–504
Clarke C, Kuruppu S, Reeve S, Ian Smith A, Hodgson WC (2006) Oxylepitoxin-1, a reversible neurotoxin from the venom of the inland taipan (Oxyuranus microlepidotus). Peptides 27:2655–2660
Davidson TM, Schafer S, Killfoil J (1995) Cobras. Wilderness Environ Med 6:203–219
Hodgson WC, Dal Belo CA, Rowan EG (2007) The neuromuscular activity of paradoxin: a presynaptic neurotoxin from the venom of the inland Taipan (Oxyuranus microlepidotus). Neuropharmacology 52:1229–1236
Kularatne SA, Budagoda BD, Gawarammana IB, Kularatne WK (2009) Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: first authenticated case series. Trans R Soc Trop Med Hyg 103:924–930
Lalloo DG, Trevett AJ, Korinhona A, Nwokolo N, Laurenson IF, Paul M, Black J, Naraqi S, Mavo B, Saweri A, Hutton RA, Theakston RDG, Warrell DA (1995) Snake bites by the Papuan taipan (Oxyuranus scutellatus canni): paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg 52:525–531
MacKay N, Ferguson JC, McNicol GP (1969) Effects of three cobra venoms on blood coagulation, platelet aggregation, and fibrinolysis. J Clin Pathol 22:304–311
Nielsen VG (2017) Crotalus atrox venom exposed to carbon monoxide has decreased fibrinogenolytic activity in vivo in rabbits. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt.12846
Nielsen VG, Bazzell CM (2016) Carbon monoxide attenuates the effects of snake venoms containing metalloproteinases with fibrinogenase or thrombin-like activity on plasmatic coagulation. MedChemComm 7:1973–1979
Nielsen VG, Bazzell CM (2017) Carbon monoxide releasing molecule-2 inhibition of snake venom thrombin-like activity: novel biochemical “brake”? J Thromb Thrombolysis 43:203–208
Nielsen VG, Garza JI (2014) Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma. Blood Coagul Fibrinolysis 25:801–805
Nielsen VG, Losada PA (2017) Direct inhibitory effects of carbon monoxide on six venoms containing fibrinogenolytic metalloproteinases. Basic Clin Pharmacol Toxicol 120:207–212
Nielsen VG, Arkebauer MR, Vosseller K (2011) Redox-based thrombelastographic method to detect carboxyhemefibrinogen-mediated hypercoagulability. Blood Coagul Fibrinolysis 22:657–661
Nielsen VG, Hafner DT, Steinbrenner EB (2013) Tobacco smoke-induced hypercoagulation in human plasma: role of carbon monoxide. Blood Coagul Fibrinolysis 24:405–410
Nielsen VG, Cerruti MA, Valencia OM, Amos Q (2016) Decreased snake venom metalloproteinase effects via inhibition of enzyme and modification of fibrinogen. Biometals 29:913–919
Nielsen VG, Sánchez EE, Redford DT (2017) Characterization of the rabbit as an in vitro and in vivo model to assess the effects of fibrinogenolytic activity of snake venom on coagulation. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt.12848
Petras D, Sanz L, Segura A, Herrera M, Villalta M, Solano D, Vargas M, León G, Warrell DA, Theakston RD, Harrison RA, Durfa N, Nasidi A, Gutiérrez JM, Calvete JJ (2011) Snake venomics of African spitting cobras: toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J Proteome Res 10:1266–1280
Speijer H, Govers-Riemslag JW, Zwaal RF, Rosing J (1986) Prothrombin activation by an activator from the venom of Oxyuranus scutellatus (Taipan snake). J Biol Chem 261:13258–13267
Storz JF, Natarajan C, Moriyama H, Hoffmann FG, Wang T, Fago A, Malte H, Overgaard J, Weber RE (2015) Oxygenation properties and isoform diversity of snake hemoglobins. Am J Physiol Regul Integr Comp Physiol 309:R1178–R1191
Wagstaff SC, Favreau P, Cheneval O, Laing GD, Wilkinson MC, Miller RL, Stöcklin R, Harrison RA (2008) Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem Biophys Res Commun 365:650–656
Funding
This investigation was supported by the Department of Anesthesiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose except that Mr. Frank is the owner of Mtoxins.
Rights and permissions
About this article
Cite this article
Nielsen, V.G., Frank, N. & Matika, R.W. Carbon monoxide inhibits hemotoxic activity of Elapidae venoms: potential role of heme. Biometals 31, 51–59 (2018). https://doi.org/10.1007/s10534-017-0066-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0066-2