Abstract
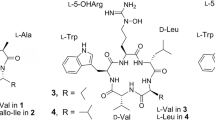
Previously, Park et al. isolated a new siderophore from Streptomyces peucetius ATCC 27952 based on information of the genome sequence and the structure of the siderophore was deduced to be a cyclic peptide based on MS/MS analysis. To clarify the structure of the siderophore, we cultured S. peucetius with iron deficient medium. Through several chromatographic procedures, the siderophore named peucechelin was isolated with the yield enough to perform NMR experiments. The planar structure of peucechelin was elucidated by the combination of ESI-MS experiment and NMR spectroscopic analyses of the gallium (III) complex. Unlike the previously deduced cyclic structure, the structure was determined to be a linear peptide which was similar to a known siderophore foroxymithine. The stereochemistries of amino acids constituting peucechelin were determined by applying modified Marfey method to the hydrolysate. Since the biosynthetic gene of peucechelin was formerly determined by Park et al. the similar genes were searched using genome data of other streptomycetes. As a result, the similar genes were found in the genome data of S. venezuelae and S. purpureus. Isolation and identification of siderophore was performed from the iron deficient culture of S. venezuelae. The siderophore of S. venezuelae was identified to be known compound foroxymithine by analysis ESI-MS and NMR spectra in the similar manner with peucechelin. Production of foroxymithine was also observed in the iron deficient culture of S. purpureus. Based on the genome data, comparison of the biosynthetic genes of structurally related siderophores peucechelin and foroxymithine was accomplished in discussion.





Similar content being viewed by others
References
Ahmad M (2011) Investigations into the isolation, structure elucidation and biosynthesis of bioactive natural products. Doctoral dissertation, University of Warwick
Ahmed E, Holmstrom SJ (2014) Siderophores in environmental research: roles and applications. Microb Biotechnol 7:196–208. doi:10.1111/1751-7915.12117
Challis GL, Ravel J (2000) Coelichelin, a new peptide siderophore encoded by the Streptomyces coelicolor genome: structure prediction from the sequence of its non-ribosomal peptide synthetase. FEMS Microbiol Lett 187:111–114
Doroghazi JR et al (2014) A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat Chem Biol 10:963–968. doi:10.1038/nchembio.1659
Harada KI, Fujii K, Hayashi K, Suzuki M, Ikai Y, Oka H (1996) Application of d, l-FDLA derivatization to determination of absolute configuration of constituent amino acids in peptide by advanced Marfey’s method. Tetrahedron Lett 37:3001–3004
Kodani S, Kayameya MO, Yoshida M, Ochi K (2011) A new siderophore isolated from Streptomyces sp. TM-34 with potent inhibitory activity against angiotensin-converting enzyme. Eur J Org Chem 17:3191–3196
Kodani S et al (2013a) Structure and biosynthesis of scabichelin, a novel tris-hydroxamate siderophore produced by the plant pathogen Streptomyces scabies 87.22. Org Biomol Chem 11:4686–4694. doi:10.1039/c3ob40536b
Kodani S, Kobayakawa F, Hidaki M (2013b) Isolation and structure determination of new siderophore tsukubachelin B from Streptomyces sp. TM-74. Nat Prod Res 27:775–781. doi:10.1080/14786419.2012.698412
Kodani S, Komaki H, Suzuki M, Hemmi H, Ohnishi-Kameyama M (2015) Isolation and structure determination of new siderophore albachelin from Amycolatopsis alba. Biometals 28:381–389. doi:10.1007/s10534-015-9842-z
Lautru S, Deeth RJ, Bailey LM, Challis GL (2005) Discovery of a new peptide natural product by Streptomyces coelicolor genome mining. Nat Chem Biol 1:265–269. doi:10.1038/nchembio731
Lazos O, Tosin M, Slusarczyk AL, Boakes S, Cortes J, Sidebottom PJ, Leadlay PF (2010) Biosynthesis of the putative siderophore erythrochelin requires unprecedented crosstalk between separate nonribosomal peptide gene clusters. Chem Biol 17:160–173. doi:10.1016/j.chembiol.2010.01.011
Park HM et al (2013) Genome-based cryptic gene discovery and functional identification of NRPS siderophore peptide in Streptomyces peucetius. Appl Microbiol Biotechnol 97:1213–1222. doi:10.1007/s00253-012-4268-9
Pullan ST, Chandra G, Bibb MJ, Merrick M (2011) Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genom 12:175. doi:10.1186/1471-2164-12-175
Robbel L, Knappe TA, Linne U, Xie X, Marahiel MA (2010) Erythrochelin: a hydroxamate-type siderophore predicted from the genome of Saccharopolyspora erythraea. FEBS J 277:663–676. doi:10.1111/j.1742-4658.2009.07512.x
Seyedsayamdost MR, Traxler MF, Zheng SL, Kolter R, Clardy J (2011) Structure and biosynthesis of amychelin, an unusual mixed-ligand siderophore from Amycolatopsis sp. AA4. J Am Chem Soc 133:11434–11437. doi:10.1021/ja203577e
Stephan H, Freund S, Meyer I, Winkelmann G, Jung C (1993) Structure elucidation of the gallium-ornibactin complex by 2D-NMR spectroscopy. Lieb Anna Chem 1993:43–48
Umezawa H et al (1985) Foroxymithine, a new inhibitor of angiotensin-converting enzyme, produced by actinomycetes. J Antibiot 38:1813–1815
Acknowledgments
This study was supported by the Japan Society for the Promotion of Science by Grants-in-aids (Grant No. 25350964).
Conflict of interest
The authors had no conflict of interest in undertaking this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kodani, S., Komaki, H., Suzuki, M. et al. Structure determination of a siderophore peucechelin from Streptomyces peucetius . Biometals 28, 791–801 (2015). https://doi.org/10.1007/s10534-015-9866-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9866-4