Abstract
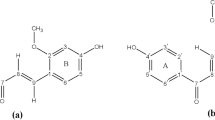
We have determined the structure and coordination chemistry of rhizoferrin (Rf), which is a particular type of siderophore, and its Fe(III) complexes using density functional theory calculations. Our results show that the Fe(III) ion binds in an octahedral coordination, with a low-spin (S = 1/2) charge-neutral chiral complex having the largest binding energy of the investigated complexes. We have also calculated nuclear magnetic resonance parameters, such as chemical shifts for 1H and 13C, and indirect nuclear spin–spin couplings for 1H–1H and 13C–1H in free Rf and in a low-spin neutral Rf metal complex, as well as nuclear quadrupole interaction parameters, such as asymmetry parameter and nuclear quadrupole coupling constants for 14N. Our calculated values for the chemical shifts for free Rf are in excellent agreement with experimental data while the calculated NMR parameters for Fe(III) complexes are predictions for future experimental work.



Similar content being viewed by others
References
Abergel RJ, Warner JA, Shuh DK, Raymond KN (2006) Enterobactin protonation and iron release: structural characterization of the salicylate coordination shift in ferric enterobactin. J Am Chem Soc 128:8920–8931
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior 38:3098–3100
Bertini I, Turano P, Vila A (1993) Nuclear magnetic resonance of paramagnetic metalloproteins. Chem Rev 93:2833–2932
Bullen JJ, Griffiths E (1987) Iron and infection—molecular, physiological and clinical aspects. Wiley, New York
Carrano CJ, Drechsel H, Kaiser D, Jung G, Matzanke B, Winkelmann G, Rochel N, AlbrechtGary AM (1996) Coordination chemistry of the carboxylate type siderophore rhizoferrin: the iron(III) complex and its metal analogs. Inorg Chem 35(22):6429–6436
Das TP, Hahn EL (1957) Nuclear quadrupole resonance spectroscopy. Academic, New York
Das TP, Hahn EL (1958) Nuclear quadrupole resonance spectroscopy. In: Seitz F, Turnbull D (eds) Solid state physics, supplement 1. Academic, New York
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724
Drechsel H, Metzger J, Freund S, Jung G, Boelaert JR, Winkelmann G (1991) Rhizoferrin—a novel siderophore from the fungus Rhizopus microsporus var. rhizopodiformis. Biol Metals 4:238–243
Drechsel H, Jung G, Winkelmann G (1992) Stereochemical characterization of rhizoferrin and identification of its dehydration products. Biometals 5:141–148
Drechsel H, Tschierske M, Thieken A, Jung G, Zahner H, Winkelmann G (1995) The carboxylate type siderophore rhizoferrin and its analogs produced by directed fermentation. J Ind Microbiol 14:105–112
Edwards DC, Myneni S (2006) Near edge X-ray absorption fine structure spectroscopy of bacterial hydroxamate siderophores in aqueous solutions. J Phys Chem A 110:11809–11818
Esteves M.A, Vaz A C T, Goncalves M L L S, Farkas E, Santos M A (1995) Siderophore analogues. Synthesis and chelating properties of a new macrocyclic trishydroxamate ligand. J Chem Soc, Dalton Trans 2565–2573
Friebolin H (1991) Basic one and two dimensional NMR spectroscopy. VCH, Weinheim
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian Inc, Wallingford
Geertsen J, Oddershede J, Raynes WT (1993) Angular dependence of geminal spin–spin coupling constants in a prototype CH2 group. J(H,H) versus interbond angle in methane. Magn Reson Chem 31(8):722–725
Goldman Domagal SD et al (2008) Quantum chemical study of the Fe(III)-desferrioxamine B siderophore complex—electronic structure, vibrational frequencies, and equilibrium Fe-isotope fractionation. Geochim Cosmochim Acta. doi:10.1016/j.gca.2008.09.031
Griffiths E (1991) Iron and bacterial virulence-a brief overview. Biol Met 4:7–13
Han S, Zaniewski RP, Marr ES, Lacey BM, Tomaras AP, Evdokimov A, Miller RJ, Shanmugasundaram V (2010) Structural basis for effectiveness of siderophore-conjugated monocarbams against clinically relevant strains of pseudomonas aeruginosa. Proc Natl Acad Sci USA 107:22002–22007
Hehre WJ, Ditchfield R, Pople JA (1972) Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56:2257
Heinisch L, Wittman S, Stoiner T, Scherlitz-Hofmann I, Ankel-Fuchs D, Möllmann U (2003) Synthesis and biological activity of tris- and tetrakiscatecholate siderophores based on poly-aza alkanoic acids or alkylbenzoic acids and their conjugates with beta-lactam antibiotics. Arzneim-Forschung Drug Res 53:188
Hilder R, Kong X (2009) Chemistry and biology of siderophores. Nat Prod Rep 27:637–657
Holinsworth B, Martin J (2009) Siderophore production by marine-derived fungi. Biometals 22:625–632
Klessinger M, Bolte P (1988) Hybridization and valence angle dependence of geminal NMR coupling constants. J Mol Struct (Theochem) 169:119
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–790
Liu J, Obando D, Schipanski LK, Witting PK, Kalinowski DS, Richardson DR, Codd R (2010) Conjugates of desferrioxamine B(DFOB) with derivatives of adamantane or with orally available chelators as potential agents for treating iron overload. J Med Chem 53:1370–1382
Maciel GE, McIver JW, Ostlund NS, Pople JA (1970) Approximate self-consistent molecular orbital theory of nuclear spin coupling. III. Geminal proton–proton coupling constants. J Am Chem Soc 92(14):4151–4157
Möllmann U, Heinisch L, Bauernfeind A, Köhler T, Ankel-Fuchs D (2009) Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22:615–624
Muller N, Pritchard DE (1959) C13 splittings in proton magnetic resonance spectra I. Hydrocarbons. J Chem Phys 31:768–771
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. I J Chem Phys 23:1833–1840
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
O’Konski CT, Ha TK (1968) Interpretation of nuclear quadrupole coupling in nitrogen containing molecules with ab initio molecular-orbital wavefunctions. J Chem Phys 49(12):5354–5361
Pierre JL, Gautier-Luneau (2000) Iron and citric acid: a fuzzy chemistry of ubiquitous biological relevance. BioMetals 13:91–96
Raghunathan K, Andriessen J, Ray SN, Das TP (1980) Explanation of nuclear quadrupole interaction in 14N “spherical” atomic ground state. Phys Rev Lett 44(5):312–315
Ramsey NF (1953) Electron coupled interactions between nuclear spins in molecules. Phys Rev 91:303
Rivault F, Liébert C, Burger A, Hoegy F, Abdallah MH, Schalk IJ, Mislin GL (2007) Synthesis of pyochelin-norfloxacin conjugates. Bioorg Med Chem Lett 17:640–644
Roosenberg JM, Lin YM, Lu Y (2000) Studies and syntheses of siderophores, microbial iron chelators, and analogs as potential drug delivery agents. Curr Med Chem 7:159–197
Tschierske M, Drechsel H, Jung G, Zahner H (1996) Production of rhizoferrin and new analogues obtained by directed fermentation. Appl Microbiol Biotechnol 45:664–670
Watanabe NA, Nagasu T, Katsu K, Kitoh K (1987) E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother 31(4):497–504
Weinberg ED (1990) Cellular iron metabolism in health and disease. Drug Metab Rev 22:531–579
Acknowledgments
Argonne National Laboratory is a US DOE Science Laboratory operated under contract no. DE-AC02-06CH11357 by UChicago Argonne, LLC. The research was performed, in part, at Argonne National Laboratory as a research participant in the Visiting Faculty Program. The program is administered by Argonne’s Division of Communication, Education, and Public Affairs (CEPA) with funding provided by the U.S. Department of Energy. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation grant number OCI-1053575. The authors acknowledge the University of Central Florida Stokes Advanced Research Computing Center for providing computational resources and support that have contributed to results reported herein. URL: http://webstokes.ist.ucf.edu.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dubey, A., Heinonen, O. First-principles electronic structure study of rhizoferrin and its Fe(III) complexes. Biometals 26, 1003–1012 (2013). https://doi.org/10.1007/s10534-013-9677-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9677-4