Abstract
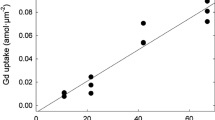
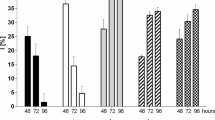
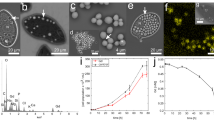
Despite 40+ years of research on aluminum (Al) toxicity in aquatic organisms, Al transport mechanisms through biological membranes, and the intracellular fate of Al once assimilated, remain poorly understood. The trivalent metal scandium shares chemical similarities with Al and, unlike Al, it has a convenient radioactive tracer (Sc-46) allowing for relatively simple measurements at environmentally relevant concentrations. Thus, we investigated the potential of Sc to substitute for Al in uptake and intracellular fate studies with the green alga Chlamydomonas reinhardtii. Short-term (<60 min) competitive uptake experiments indicated that Al does not inhibit Sc influx, implying that these metals do not share a common transport mechanism. Also, internalized Al concentrations were ~4 times higher than Sc concentrations after long-term (72 h) exposures under similar conditions (4.5 μM AlT or ScT, 380 μM FT, pH 7.0, 3.8 pM Al 3+calc and 1.0 pM Sc 3+calc ). However, interesting similarities were observed in their relative subcellular distributions, suggesting possible common toxicity/tolerance mechanisms. Both metals mostly distributed to the organelles fraction and almost no association was found with the cytosolic proteins. The greatest difference was observed in the cellular debris fraction (membranes and nucleus) where Al was much more concentrated than Sc. However, it is not clear whether or not this fraction contained extracellular metal associated with the algal surface. To summarize, Sc does not seem to be an adequate substitute of Al for transport/uptake studies, but could be for investigations of toxicity/tolerance mechanisms in C. reinhardtii. Further work is needed to verify this latter suggestion.


Similar content being viewed by others
References
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York, pp 45–102
Campbell PGC, Fortin C (2013) Biotic ligand model. In: Férard J-F, Blaise C (eds) Encyclopedia of aquatic ecotoxicology. Springer, Dordrecht, Netherlands, pp 237–246. doi:10.1007/978-94-007-5704-2
Campbell PGC, Hare L (2009) Metal detoxification in freshwater animals. roles of metallothioneins. In: Metallothioneins and related chelators: metal ions in life sciences, vol 5. The Royal Society of Chemistry, London, pp 239–277. doi:10.1039/9781847559531-00239
Clarkson DT, Sanderson J (1969) The uptake of a polyvalent cation and its distribution in the root apices of Allium cepa: tracer and autoradiographic studies. Planta 89:136–154. doi:10.1007/BF00386981
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832. doi:10.1104/pp.123.3.825
Crémazy A, Campbell PGC, Fortin C (2013) The biotic ligand model can successfully predict the uptake of a trivalent ion by a unicellular alga below pH 6.50 but not above: possible role of hydroxo-species. Environ Sci Technol 47:2408–2415. doi:10.1021/es3038388
Dobson CB, Day JP, King SJ, Itzhaki RF (1998) Location of aluminium and gallium in human neuroblastoma cells treated with metal-chelating agent complexes. Toxicol Appl Pharmacol 152:145–152. doi:10.1006/taap.1998.8489
Eckhardt U, Buckhout TJ (1998) Iron assimilation in Chlamydomonas reinhardtii involves ferric reduction and is similar to Strategy I higher plants. J Exp Bot 49:1219–1226. doi:10.1093/jexbot/49.324.1219
Fortin C, Dutel L, Garnier-Laplace J (2004) Uranium complexation and uptake by a green alga in relation to chemical speciation: the importance of the free uranyl ion. Environ Toxicol Chem 23:974–981. doi:10.1897/03-90
Gensemer RW, Playle RC (1999) The bioavailability and toxicity of aluminum in aquatic environments. Crit Rev Environ Sci Technol 29:315–450. doi:10.1080/10643389991259245
Goulet RR, Lalonde JD, Munger C, Dupuis S, Dumont-Frenette G, Prémont S, Campbell PGC (2005) Phytoremediation of effluents from aluminum smelters: a study of Al retention in mesocosms containing aquatic plants. Water Res 39:2291–2300. doi:10.1016/j.watres.2005.04.029
Hassler CS, Slaveykova VI, Wilkinson KJ (2004) Discriminating between intra- and extracellular metals using chemical extractions. Limnol Oceanogr 2:237–247. doi:10.4319/lom.2004.2.237
Hem JD, Roberson CE (1967) Form and stability of aluminum hydroxide complexes in dilute solution. U.S. G.P.O, Washington
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197. doi:10.1093/aob/mcq053
Kinraide TB (1994) Use of Gouy–Chapman–Stern model for membrane–surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiol 106:1583–1592. doi:10.1104/pp.106.4.1583
Kiss T (1995) Interaction of aluminum with biomolecules—any relevance to Alzheimer’s disease? Arch Gerontol Geriatr 21:99–112. doi:10.1016/0167-4943(95)00642-X
Kobayashi S, Nagayama S, Busujima T (1998) Lewis acid catalysts stable in water. Correlation between catalytic activity in water and hydrolysis constants and exchange rate constants for substitution of inner-sphere water ligands. J Am Chem Soc 120:8287–8288. doi:10.1021/Ja980715q
Komine Y, Eggink LL, Park H, Hoober JK (2000) Vacuolar granules in Chlamydomonas reinhardtii: polyphosphate and a 70-kDa polypeptide as major components. Planta 210:897–905. doi:10.1007/s004250050695
Lavoie M, Le Faucheur S, Fortin C, Campbell PGC (2009) Cadmium detoxification strategies in two phytoplankton species: metal binding by newly synthesized thiolated peptides and metal sequestration in granules. Aquat Toxicol 92:65–75. doi:10.1016/j.aquatox.2008.12.007
Lavoie M, Le Faucheur S, Fortin C, Campbell PGC (2011) Erratum to “Cadmium detoxification strategies in two phytoplankton species: metal binding by newly synthesized thiolated peptides and metal sequestration in granules”. Aquat Toxicol 101:298 (Aquat Toxicol 92 (2009) 65–75). doi:10.1016/j.aquatox.2010.09.015
Li L, Tutone AF, Drummond RSM, Gardner RC, Luan S (2001) A novel family of magnesium transport genes in Arabidopsis. Plant Cell 13:2761–2775. doi:10.1105/tpc.010352
Lindqvist-Reis P, Persson I, Sandstrom M (2006) The hydration of the scandium(III) ion in aqueous solution and crystalline hydrates studied by XAFS spectroscopy, large-angle X-ray scattering and crystallography. Dalton Trans 28(32):3868–3878. doi:10.1039/b604267h
MacDiarmid CW, Gardner RC (1998) Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem 273:1727–1732. doi:10.1074/jbc.273.3.1727
Martell AE, Smith RM, Motekaitis RJ (1997) Critical stability constants of metal complexes database [NIST Standard Reference Database 46] version 4.0. US Departement of Commerce, Gaithersburg, MD
Martin RB (1986) The chemistry of aluminum as related to biology and medicine. Clin Chem 32:1797–1806
Martin RB (1992) Aluminium speciation in biology. In: Chadwick DJ, Whelan J (eds) Aluminium in biology and medicine. Wiley, New York, pp 5–25. doi:10.1002/9780470514306.ch2
Matsumoto H (1991) Biochemical mechanism of the toxicity of aluminium and the sequestration of aluminium in plant cells. Dev Plant Soil Sci 45:825–838
Moomaw AS, Maguire ME (2010) Cation selectivity by the CorA Mg2+ channel requires a fully hydrated cation. Biochemistry 49:5998–6008. doi:10.1021/bi1005656
Nordstrom DK, Plummer LN, Langmuir D, Busenberg E, May HM, Jones BF, Parkhurst DL (1990) Revised chemical equilibrium data for major water—mineral reactions and their limitations. In: Melchior DC, Bassett RL (eds) Chemical modeling of aqueous systems II, vol 416. Acs symposium series. American Chemical Society, Washington, pp 398–413
Pettersson A, Bergman B (1989) Effects of aluminium on ATP pools and utilization in the cyanobacterium Anabaena cylindrica: a model for the in vivo toxicity. Physiol Plant 76:527–534. doi:10.1111/j.1399-3054.1989.tb05473.x
Pettersson A, Kunst L, Bergman B, Roomans GM (1985) Accumulation of aluminum by Anabaena cylindrica into polyphosphate granules and cell walls: an X-ray energy-disperse microanalysis study. J Gen Microbiol 131:2545–2548. doi:10.1099/00221287-131-10-2545
Rengel Z (1996) Uptake of aluminium by plant cells. New Phytol 134:389–406. doi:10.1111/j.1469-8137.1996.tb04356.x
Rengel Z, Reid RJ (1997) Uptake of Al across the plasma membrane of plant cells. Plant Soil 192:31–35. doi:10.1023/A:1004265913770
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr 32:751–767. doi:10.1107/S0567739476001551
Silva IR, Smyth TJ, Moxley DF, Carter TE, Allen NS, Rufty TW (2000) Aluminum accumulation at nuclei of cells in the root tip. Fluorescence detection using lumogallion and confocal laser scanning microscopy. Plant Physiol 123:543–552. doi:10.1104/pp.123.2.543
Sparling DW, Lowe TP (1996) Environmental hazards of aluminum to plants, invertebrates, fish, and wildlife. Rev Environ Contam Toxicol 145:1–127. doi:10.1007/978-1-4612-2354-2_1
Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ (2000) Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol 123:987–996. doi:10.1104/pp.123.3.987
Verstraeten SV, Oteiza PI (1995) Sc3+, Ga3+, In3+, Y3+, and Be2+ promote changes in membrane physical properties and facilitate Fe2+-initiated lipid peroxydation. Arch Biochem Biophys 322:284–290. doi:10.1006/abbi.1995.1464
Viola RE, Morrison JF, Cleland WW (1980) Interaction of metal(III)-adenosine 5′-triphosphate complexes with yeast hexokinase. Biochem 19:3131–3137. doi:10.1021/bi00555a003
Vitorello VA, Capaldi FR, Stefanuto VA (2005) Recent advances in aluminum toxicity and resistance in higher plants. Braz J Plant Physiol 17:129–143. doi:10.1006/abbi.1995.1464
Walton RC, White KN, Livens F, McCrohan CR (2010) The suitability of gallium as a substitute for aluminum in tracing experiments. Biometals 23:221–230. doi:10.1007/s10534-009-9280-x
Wilkinson KJ, Buffle J (2004) Critical evaluation of the physicochemical parameters and processes for modelling the biological uptake of trace metals in environmental (aquatic) systems. In: van Leeuwen HP, Köster W (eds) Physicochemical kinetics and transport at biointerfaces. IUPAC series on analytical and physical chemistry of environmental systems, vol 9. Wiley, Chichester, pp 445–533. doi:10.1002/0470094044.ch10
Williams RJP (2002) Recent aspects of aluminum chemistry and biology: a survey. Coord Chem Rev 228:93–96. doi:10.1016/S0010-8545(02)00072-3
Wolterbeek HT, Verburg TG (2001) Predicting metal toxicity revisited: general properties vs. specific effects. Sci Total Environ 279:87–115. doi:S0048-9697(01)00756-2
Wood SA, Samson IM (2006) The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol Rev 28:57–102. doi:10.1016/j.oregeorev.2003.06.002
Worms IAM, Wilkinson KJ (2007) Ni uptake by a green alga. 2. Validation of equilibrium models for competition effects. Environ Sci Technol 41:4264–4270. doi:10.1021/es0630341
Xia J, Yamaji N, Kasai T, Ma JF (2010) Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci 107:18381–18385. doi:10.1073/pnas.1004949107
Zheng SJ, Yang JL (2005) Target sites of aluminum phytotoxicity. Biol Plant 49:321–331. doi:10.1007/s10535-005-0001-1
Acknowledgments
Financial support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Rio Tinto Alcan. C. Fortin and P.G.C. Campbell are supported by the Canada Research Chair program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crémazy, A., Levy, J.L., Campbell, P.G.C. et al. Uptake and subcellular partitioning of trivalent metals in a green alga: comparison between Al and Sc. Biometals 26, 989–1001 (2013). https://doi.org/10.1007/s10534-013-9675-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9675-6