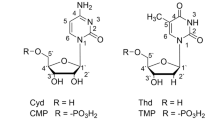
Abstract
Coordinating properties of uridine 5′-monophosphate (UMP) towards trivalent La, Pr, Nd, Sm, Eu and Gd ions in presence of cationic and anionic micelles have been investigated by potentiometric pH-titration and spectroscopic methods. Stability constants of the 2:1 complexes have been determined and the change in free energy, enthalpy and entropy associated with the complexation are also calculated. Nd(III) complexes isolated from aqueous and aqueous-micellar media do not show any significant structural difference. Formation of Ln(III) complexes in all cases completes below pH 7.5 showing that UMP best interacts with Ln3+ ions at the physiological pH range 7.3–7.5. The nucleobase is not involved in the complexation and the metal ion coordination of UMP is through the phosphate moiety only. Coordinating tendency of UMP with lanthanides, Nd(III) ion in particular, at different pH is also discussed. Luminescent properties of Eu(III) complex and its decay lifetime are also presented. This information may prove helpful regarding the use of lanthanides as biological probes for calcium/magnesium ions.









Similar content being viewed by others
References
Aime S, Botta M, Fasano M, Terreno E (1998) Lanthanide(III) chelates for NMR biomedical applications. Chem Soc Rev 27:19–29
Azab HA, Anwar ZM, Ahmed RG (2010) Pyrimidine and purine mononucleotides recognition by trivalent lanthanide complexes with N-acetyl amino acids. J Chem Eng Data 55:459–475
Azab HA, Al-Deyab SS, Anwar ZM, Ahmed RG (2011) Fluorescence and electrochemical probing of N-acetylamino acids, nucleotides, and DNA by the Eu(III)–Bathophenanthroline complex. J Chem Eng Data 56:833–849
Bianchi EM, Sajadi SAA, Song B, Sigel H (2003) Stabilities and isomeric equilibria in aqueous solution of monomeric metal ion complexes of adenosine 5′-diphosphate(ADP3−) in comparison with those of adenosine 5′-monophosphate(AMP2−). Chem Eur J 9(4):881–892
Bjerrum J (1941) Metal ammine formation in aqueous solution. Hass and Sons, Copenhagen
Brown PK, Gibbons IR, Wald G (1963) The visual cells and visual pigment of mudpuppy, necturus. J Cell Biol 19:79–106
Buono-Core GE, Li H, Marciniak B (1990) Quenching of excited states by lanthanide ions and chelates in solution. Coord Chem Rev 99:55–87
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB (1999) Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev 99:2293–2352
Costa CPD, Sigel H (1999) Stabilities of complexes formed between lead(II) and simple phosphate or phosphate monoester ligands including some pyrimidine-nucleoside 5′-monophosphates (CMP2−, UMP2−, dTMP2−). J Biol Inorg Chem 4:508–514
Das AK (2004) Micellar effect on the kinetics and mechanism of chromium(VI) oxidation of organic substrates. Coord Chem Rev 248:81–99
Dunlap RB, Cordes EH (1968) Secondary valance force catalysis. VI. Catalysis of hydrolysis of methyl orthobenzoate by sodium dodecyl sulfate. J Am Chem Soc 90:4395–4404
Elbanowski M, Makowska B (1996) The lanthanides as luminescent probes in investigations of biochemical systems. J Photochem Photobiol A Chem 99:85–92
Fricker SP (2006) The therapeutic application of lanthanides. Chem Soc Rev 35:524–533
Fugate A, Coppin GR (2003) Application of the Judd-Ofelt theory in lanthanide-chelidamic acid complexation. Mol Phys 101(7):935–939
Herrero LA, Terron A (2000) Interaction in solution of calcium(II) and copper(II) with nucleoside monophosphates: a calorimetric study. J Biol Inorg Chem 5:269–275
Horrocks DeW Jr, Collier WE (1981) Lanthanide ion luminescence probes. Measurement of distance between intrinsic protein fluorophores and bound metal ions: quantitation of energy transfer between tryptophan and terbium(III) or europium(III) in the calcium-binding protein parvalbumin. J Am Chem Soc 103(10):2856–2862
Horrocks WDeW Jr, Sudnick DR (1979) Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J Am Chem Soc 101(2):334–340
Ilmi R, Iftikhar K (2012) Luminescent nine coordinated complexes derived from fluorinated β-diketone and 2,4,6-tris(2-pyridyl)-1,3,5-triazine. J Coord Chem 65(3):403–419
Imamoto T (1994) Lanthanides in organic synthesis. Academic Press, London
Irving HM, Rossotti HS (1953) Methods for computing successive stability constants from experimental formation curves. J Chem Soc 3397–3405
Izatt RM, Christensen JJ, Rytting JH (1971) Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem Rev 71(5):439–481
Komiyama M, Takeda N, Shigekawa H (1999) Hydrolysis of DNA and RNA by lanthanide ions: mechanistic studies leading to new applications. Chem Commun 1443–1451
Lavanya KV, Kumar KYK, Rao TS, Rao GN (2003) Micellar effect on metal–ligand equilibria of magnesium(II) and calcium(II) with l-arginine. J Indian Chem Soc 80:783–786
Lonibala RK, Sudhiranjan Singh M, Homendra N (2009) Effect of nonionic micelles on the stability of the complexes of uridine 5′-monophosphate with lighter lanthanide ions in aqueous media. J Surf Sci Technol 25(3–4):189–202
Massoud SS, Sigel H (1988) Metal ion coordinating properties of pyrimidine-nucleoside 5′-monophosphates (CMP, UMP, TMP) and simple phosphate monoesters, including D-ribose 5′-monophosphate. Establishment of relations between complex stability and phosphate basicity. Inorg Chem 27:1447–1453
Menger FM, Portnoy CE (1967) On the chemistry of reaction proceeding inside molecular aggregates. J Am Chem Soc 89(18):4698–4703
Orabi AS, Azab HA, Saad F, Said H (2010) Ternary complexes of La(III), Ce(III), Pr(III) or Er(III) with adenosine 5′-mono, 5′-di and 5′-triphosphate as primary ligands and some biologically important zwitterionic buffers as secondary ligands. J Solut Chem 39:319–334
Parker D (1991) NMR determination of enantiomeric purity. Chem Rev 91(7):1441–1457
Parker D (2000) Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord Chem Rev 205:109–130
Phillips R (1966) Adenosine and adenine nucleotides: ionization, metal complex formation and conformation in solution. Chem Rev 66:501–527
Romsted LS, Cordes EH (1968) Secondary valance force catalysis. VII. Catalysis of hydrolysis of p-nitrophenyl hexanoate by micelle-forming cationic detergents. J Am Chem Soc 90:4404–4409
Rosen MJ (1998) Surfactants and interfacial phenomena, vol 2. Wiley, New York
Sabbatini N, Guardigli M (1993) Luminescent lanthanide complexes as photochemical supramolecular devices. Coord Chem Rev 123:201–228
Sagar VUS, Himabindu G, Sudarsan KG, Rao GN (2005) Speciation studies of binary complexes of Nickel(II) with l-arginine and l-histidine in micellar medium. J Indian Chem Soc 82:589–601
Sigel H (1993) Interaction of metal ions with nucleotides and nucleic acids and their constituents. Chem Soc Rev 22:255–267
Sigel H, Griesser R (2009) Nucleoside 5′-triphosphate: self association, acid-base, and metal ion binding properties in solution. Chem Soc Rev 34:875–900
Tajmir-Riahi HA, Theophanides T (1983) Adenosine-5′-monophosphate complexes of Pt(II) and Mg(II) metal ions. Synthesis, FTIR spectra and structural studies. Inorg Chim Acta 80:183–190
Tascioglu S (1996) Micellar solutions as reaction media. Tetrahedron 52(34):11113–11152
Tribolet R, Malini-Balakrishnan R, Sigel H (1985) Influence of solvent polarity (dioxane water mixtures) on the stability and structure of binary and ternary complexes of adenosine 5′-triphosphate and Uridine 5′-triphosphate. J Chem Soc Dalton Trans 2291–2303
Umebayashi Y, Nagahama Y, Ishiguro S (1997) Unusual behavior of thiocyanato complexation with copper(II) and zzinc(II) ions in micellar solution of a non-ionic surfactant TritonX-100. J Chem Soc Faraday Trans 93(7):1377–1381
Umebayashi Y, Shin M, Kanzaki R, Ishiguro S (2006) Metal ion complexes in surfactant solution. Encyclopedia of surface and colloid science, vol 2. Marcel Dekker, New York
Van Pelt GCE (1993) Studies of lanthanide interactions with polyoxometalates. PhD Thesis, Florida state University, Tallahassee
Vold RD, Vold MJ (1983) Colloid and interface chemistry. Addisson-Wesley, London
Yamauchi O, Odani A, Masuda H, Sigel H (1996) Interaction of metal ions with nucleotides, nucleic acids and their constituents. Metal ions in biological systems, vol 32. Marcel Dekker, New York, pp 207–270
Zhu B, Wu YJ, Zhao DQ, Ni JZ (1999) Cleavage of nucleotides by Ce4+ and the lanthanide metal complexes. BioMetals 12:11–17
Acknowledgments
This research was financed by Department of Science & Technology, New Delhi under Scheme No. SR/SI/PC-39/2003 dated 19/03/2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sudhiranjan Singh, M., Homendra, N. & Lonibala, R.K. Coordinating properties of uridine 5′-monophosphate with selected Ln3+ ions in ionic micellar media. Biometals 25, 1235–1246 (2012). https://doi.org/10.1007/s10534-012-9585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-012-9585-z