Abstract
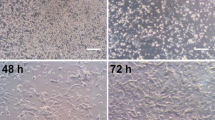
Selenium (Se) and Selenoprotein W (SelW) plays a pivotal role in the brain development, function, and degeneration and that SelW expression in the brain may be affected by Se. However, the mechanism which Se regulates the SelW gene expression in neurons remains to be unclear. To investigate the effects of the SelW gene expression and mRNA stability induced by Se, primary cultured chicken embryos neurons derived from 8-day-old chick embryo cerebral hemispheres were treated with 10−9–10−5 mol/l Se as selenite for 3, 6, 12, 24 or 48 h, respectively. The morphology and viability of Neurons was detected. The SelW mRNA expression level and mRNA half-life was examined in Se-treated neurons. The relative low concentrations of Se enhanced the neurite outgrowth, increased the SelW mRNA levels and elevated the mRNA half-life of chick embryo neurons. In contrast, the high concentrations of Se presented neurotoxic to neurons, decreased the SelW mRNA levels and reduced the mRNA half-life of neuronal cells. These results suggest that the alteration of post-transcriptional stabilization of SelW mRNA is an important mechanism of Se-induced the elevation or reduction of the SelW expression level in chick embryo neurons.




Similar content being viewed by others
References
Allmang C, Wurth L, Krol A (2009) The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta 1790(11):1415–1423
Amantana A, Vorachek WR, Butler JA, Costa ND, Whanger PD (2002) Effect of copper, zinc and cadmium on the promoter of selenoprotein W in glial and myoblast cells. J Inorg Biochem 91(2):356–362
Bellingham J, Gregory-Evans K, Fox MF, Gregory-Evans CY (2003) Gene structure and tissue expression of human selenoprotein W, SEPW1, and identification of a retroprocessed pseudogene, SEPW1P. Biochim Biophys Acta 1627(2–3):140–146
Bermano G, Arthur JR, Hesketh JE (1996) Selective control of cytosolic glutathione peroxidase and pholipid hydroperoxide glutathione peroxidase mRNA stability by selenium supply. FEBS Lett 387(2–3):157–160
Blanquicett C, Kang BY, Ritzenthaler JD, Jones DP, Hart CM (2010) Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic Biol Med 48(12):1618–1625
Brauer AU, Savaskan NE (2004) Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev Neurosci 15(1):19–32
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86(1):1–12
Christensen MJ, Burgener KW (1992) Dietary selenium stabilizes glutathione peroxidase mRNA in rat liver. J Nutr 122(8):1620–1626
Chung YW, Jeong D, Noh OJ, Park YH, Kang SI, Lee MG et al (2009) Antioxidative role of selenoprotein W in oxidant-induced mouse embryonic neuronal cell death. Mol Cells 27(5):609–613
Cline HT (2001) Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11(1):118–126
De Rubeis S, Bagni C (2010) Fragile × mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability. Mol Cell Neurosci 43(1):43–50
Gromer S, Eubel JK, Lee BL, Jacob J (2005) Human selenoproteins at a glance. Cell Mol Life Sci 62(21):2414–2437
Gu QP, Sun Y, Ream LW, Whanger PD (2000) Selenoprotein W accumulates primarily in primate skeletal muscle, heart, brain and tongue. Mol Cell Biochem 204(1–2):49–56
Gu QP, Ream W, Whanger PD (2002) Selenoprotein W gene regulation by selenium in L8 cells. Biometals 15(4):411–420
Gwon AR, Park JS, Park JH, Baik SH, Jeong HY, Hyun DH et al (2010) Selenium attenuates A beta production and A beta-induced neuronal death. Neurosci Lett 469(3):391–395
Himeno S, Imura N (2002) Selenium in nutrition and toxicology. In: Sarkar B (ed) Heavy metals in the environment, 1st edn. CRC Press, New York, pp 614–618
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Hoppe B, Brauer AU, Kuhbacher M, Savaskan NE, Behne D, Kyriakopoulos A (2008) Biochemical analysis of selenoprotein expression in brain cell lines and in distinct brain regions. Cell Tissue Res 332(3):403–414
Jeong DW, Kim EH, Kim TS, Chung YW, Kim H, Kim IY (2004) Different distributions of selenoprotein W and thioredoxin during postnatal brain development and embryogenesis. Mol Cells 17(1):156–159
Kim YJ, Chai YG, Ryu JC (2005) Selenoprotein W as molecular target of methylmercury in human neuronal cells is down-regulated by GSH depletion. Biochem Biophys Res Commun 330(4):1095–1102
Li JL, Gao R, Li S, Wang JT, Tang ZX, Xu SW (2010) Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23(4):695–705
Li JL, Li HX, Li S, Jiang ZH, Xu SW, Tang ZX (2011a) Selenoprotein W gene expression in the gastrointestinal tract of chicken is affected by dietary selenium. Biometals 24(2):291–299. doi:10.1007/s10534-010-9395-0
Li JL, Ruan HF, Li HX, Li S, Xu SW, Tang ZX (2011b) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian Selenoprotein W from chicken. Mol Biol Rep 38(6):4015–4022. doi:10.1007/s11033-010-0520-5
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100(10):1679–1684
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284(2):723–727
Mangoura D, Dawson G (1993) Opioid peptides activate phospholipase D and protein kinase C-epsilon in chicken embryo neuron cultures. Proc Natl Acad Sci USA 90(7):2915–2919
Nakayama A, Hill KE, Austin LM, Motley AK, Burk RF (2007) All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J Nutr 137(3):690–693
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31(14):e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Poirazi P, Mel BW (2001) Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29(3):779–796
Ralston NV, Ralston CR, Blackwell JL 3rd, Raymond LJ (2008) Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 29(5):802–811
Savaskan NE, Brauer AU, Kuhbacher M, Eyupoglu IY, Kyriakopoulos A, Ninnemann O et al (2003) Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J 17(1):112–114
Schweizer U, Schomburg L (2006) Selenium, selenoproteins and brain function. In: Hatfield DL, Berry MJ, Gladyshev VN (eds) Selenium: its molecular biology and role in human health. Springer, New York, pp 233–248
Schweizer U, Schomburg L, Savaskan NE (2004a) The neurobiology of selenium: lessons from transgenic mice. J Nutr 134(4):707–710
Schweizer U, Brauer AU, Kohrle J, Nitsch R, Savaskan NE (2004b) Selenium and brain function: a poorly recognized liaison. Brain Res Brain Res Rev 45(3):164–178
Selenius M, Rundlof AK, Olm E, Fernandes AP, Bjornstedt M (2010) Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid Redox Signal 12(7):867–880
Sun Y, Ha PC, Butler JA, Ou BR, Yeh JY, Whanger PD (1998) Effect of dietary selenium on selenoprotein W and glutathione peroxidase in 28 tissues of rat. J Nutr Biochem 9:23–27
Sun Y, Forsberg N, Whanger PD (1999) Selenoprotein W, selenium and glutathione peroxidase in rat and sheep brains and in brain cell cultures. Nutr Neurosci 2:227–237
Sun Y, Gu QP, Whanger PD (2001a) Selenoprotein W in overexpressed and underexpressed rat glial cells in culture. J Inorg Biochem 84(1–2):151–156
Sun Y, Butler JA, Whanger PD (2001b) Glutathione peroxidase activity and selenoprotein W levels in different brain regions of selenium-depleted rats. J Nutr Biochem 12(2):88–94
Vendeland SC, Beilstein MA, Chen CL, Jensen ON, Barofsky E, Whanger PD (1993) Purification and properties of selenoprotein W from rat muscle. J Biol Chem 268(23):17103–17107
Whanger PD (2000) Selenoprotein W: a review. Cell Mol Life Sci 57(13–14):1846–1852
Whanger PD (2001) Selenium and the brain: a review. Nutr Neurosci 4(2):81–97
Whanger PD (2009) Selenoprotein expression and function—selenoprotein W. Biochim Biophys Acta 1790(11):1448–1452
Xiao R, Qiao JT, Zhao HF, Liang J, Yu HL, Liu J et al (2006) Sodium selenite induces apoptosis in cultured cortical neurons with special concomitant changes in expression of the apoptosis-related genes. Neurotoxicology 27(4):478–484
Yeh JY, Beilstein MA, Andrews JS, Whanger PD (1995) Tissue distribution and influence of selenium status on levels of selenoprotein W. FASEB J 9(5):392–396
Yeh JY, Vendeland SC, Gu Q, Butler JA, Ou BR, Whanger PD (1997) Dietary selenium increases selenoprotein W levels in rat tissues. J Nutr 127(11):2165–2172
Zeng H, Combs GF Jr (2008) Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem 19(1):1–7
Zhang Y, Zhou Y, Schweizer U, Savaskan NE, Hua D, Kipnis J et al (2008) Comparative analysis of selenocysteine machinery and selenoproteome gene expression in mouse brain identifies neurons as key functional sites of selenium in mammals. J Biol Chem 283(4):2427–2438
Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ et al (2009) Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr 139(6):1061–1066
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 30871902) and the Science Foundation of the Education Department of Heilongjiang Province (Grant No. 11551030).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Effects of Se and incubation period on the expression of GADPH mRNA in chicken embryo neurons. GADPH mRNA expression level in neurons was measured by quantitative real-time RT-PCR. Bars represent mean ± standard deviation of triplicate cultures. (PPT 165 kb)
Fig. S2
Effects of Se on the half-life of SelW mRNA in chicken embryo neurons. The chicken embryo neuron monolayers were treated with 0 mol/l, ActD, ActD + 10−8 mol/l Se, ActD + 10−7 mol/l Se, ActD + 10−6 mol/l Se or ActD + 10−5 mol/l Se for 0 h, 3 h, 6 h, 9 h, 12 h, 24 h or 48 h, respectively. SelW mRNA expression level in chicken embryo neurons was measured by quantitative real-time RT-PCR. The RNA half-life was extrapolated from the SelW mRNA decay curve as the time point after 5μg/mL ActD treatment. (PPT 367 kb)
Rights and permissions
About this article
Cite this article
Li, JL., Li, HX., Li, S. et al. Effects of Selenoprotein W gene expression by selenium involves regulation of mRNA stability in chicken embryos neurons. Biometals 25, 459–468 (2012). https://doi.org/10.1007/s10534-012-9517-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-012-9517-y