Abstract
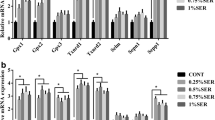
Selenium (Se), selenoprotein N (SelN) and selenoprotein W (SelW) play a crucial role in muscle disorders. Se status highly regulates selenoprotein mRNA levels. However, few attempts have been performed on the effect of dietary Se supplementation on muscle SelN and SelW mRNA levels in birds. To investigate the effects of Se on the regulation of SelN and SelW mRNA levels in muscle tissues, one-day-old male chickens were fed either a commercial diet or a Se-supplemented diet containing 1.0, 2.0, 3.0 or 5.0 mg/kg sodium selenite for 90 days. Muscle tissues (breast, flight, thigh, shank and cardiac muscles) were collected and examined for Se content and mRNA levels of SelN and SelW. Moreover, Selenophosphate synthetase-1 (SPS-1) and selenocysteine-synthase (SecS) mRNA levels were analyzed. Significant increases in SelN mRNA levels were obtained in breast, thigh and shank muscles treated with Se, with maximal effects at 3.0 mg Se/kg diet, but 2.0 mg Se/kg diet resulted in peak levels of Sel N mRNA in flight muscles. Changes in SelW mRNA abundance in thigh and shank muscles increased in response to Se supply. After reaching a maximal level, higher Se supplementation led to a reduction in both SelN and SelW mRNAs. However, SelN and SelW mRNA levels displayed a different expression pattern in different skeletal and cardiac muscles. Thus, it suggested that skeletal and cardiac muscles SelN and SelW mRNA levels were highly regulated by Se supplementation and different muscle tissues showed differential sensitivity. Moreover, Se supplementation also regulated the levels of SPS1 and SecS mRNAs. The mRNA levels of SPS1 and SecS were enhanced in the Se supplemented groups. These data indicate that Se regulates the expression of SelN and SelW gene and affect the mRNA levels of SecS and SPS1.








Similar content being viewed by others
References
Allamand V, Richard P, Lescure A, Ledeuil C, Desjardin D, Petit N, Gartioux C, Ferreiro A, Krol A, Pellegrini N, Urtizberea JA, Guicheney P (2006) A single homozygous point mutation in a 3′ untranslated region motif of selenoprotein N mRNA causes SEPN1 related myopathy. EMBO Rep 7(4):450–454
Allan CB, Lacourciere GM, Stadtman TC (1999) Responsiveness of selenoproteins to dietary selenium. Annu Rev Nutr 19:1–16
Clarke NF, Kidson W, Quijano Roy S, Estournet B, Ferreiro A, Guicheney P, Manson JI, Kornberg AJ, Shield LK, North KN (2006) SEPN1: associated with congenital fiber type disproportion and insulin resistance. Ann Neurol 59(3):546–552
Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A (2007) Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res 313(1):156–167
Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N, Bonnemann C, Jungbluth H, Straub V, Villanova M, Leroy JP, Romero NB, Martin JJ, Muntoni F, Voit T, Estournet B, Richard P, Fardeau M, Guicheney P (2002) Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet 71(4):739–749
Ferreiro A, Ceuterick de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, Fardeau M, Martin JJ, Goebel HH, Richard P, Guicheney P, Bonnemann CG (2004) Desmin relatedmyopathy with Mallory body like inclusions is caused by mutations of the selenoprotein N gene. Ann Neurol 55(5):676–686
Gao X, Xing H, Li S, Li J, Ying T, Xu S (2011) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol Trace Elem Res (in press) doi:10.1007/s12011-011-9175-x
Hasunuma R, Ogawa T, Kawanishi Y (1982) Fluorometric determination of selenium in nanogram amounts in biological materials using 2,3-diaminonaphthalene. Anal Biochem 126(2):242–245
Hatfield DL, Carlson BA, Xu XM, Mix H, Gladyshev VN (2006) Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog Nucleic Acid Res Mol Biol 81:97–142
Hendrickson TL (2007) Easing selenocysteine into proteins. Nat Struct Mol Biol 14:100–101
Irons R, Carlson BA, Hatfield DL, Davis CD (2006) Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr 136:1311–1317
Jurynec MJ, Xia R, Mackrill JJ, Gunther D, Crawford T, Flanigan KM et al (2008) Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci USA 105(34):12485–12490
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Lescure A, Gautheret D, Carbon P, Krol A (1999) Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J Biol Chem 274(53):38147–38154
Li JL, Li HX, Li S, Jiang ZH, Xu SW, Tang ZX (2010) Selenoprotein W gene expression in the gastrointestinal tract of chicken is affected by dietary selenium. Biometals 24(2):291–299
Li JL, Ruan HF, Li HX, Li S, Xu SW, Tang ZX (2011) Molecular cloning, characterization and mRNA expression analysis of a novel selenoprotein: avian selenoprotein W from chicken. Mol Biol Rep 38(6):4015–4022
Lobanov AV, Hatfield DL, Gladyshev VN (2008) Selenoproteinless animals: selenophosphate synthetase SPS1 functions in a pathway unrelated to selenocysteine biosynthesis. Protein Sci 17(1):176–182
Loflin J, Lopez N, Whanger PD, Kioussi C (2006) Selenoprotein W during development and oxidative stress. J Inorg Biochem 100:1679–1684
Low SC, Harney JW, Berry MJ (1995) Cloning and functional characterization of human selenophosphate synthetase, an essential component of selenoprotein synthesis. J Biol Chem 270:21659–21664
Maiti B, Arbogast S, Allamand V, Moyle MW, Anderson CB, Richard P, Guicheney P, Ferreiro A, Flanigan KM, Howard MT (2008) A mutation in the SEPN1 selenocysteine redefinition element SRE reduces selenocysteine incorporation and leads to SEPN1 related myopathy. Human Mutat 30(3):411–416
Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D, Muntoni F, Topaloglu H, Guicheney P (2001) Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet 29(1):17–18
Okamoto Y, Takashima H, Higuchi I, Matsuyama W, Suehara M, Nishihira Y, Hashiguchi A, Hirano R, Ng AR, Nakagawa M, Izumo S, Osame M, Arimura K (2006) Molecular mechanism of rigid spine with muscular dystrophy type 1 caused by novel mutations of selenoprotein N gene. Neurogenetics 7:175–183
Pagmantidis V, Bermano G, Villette S, Broom I, Arthur J, Hesketh J (2005) Effects of Se-depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett 579(3):792–796
Petit N, Lescure A, Rederstorff M, Krol A, Moghadaszadeh B, Wewer UM, Guicheney P (2003) Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum Mol Genet 12:1045–1053
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Raines AM, Sunde RA (2011) Selenium toxicity but not deficient or supernutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics 12:26
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63(1):52–59
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66(15):2457–2478
Ruan H, Zhang Z, Wu Q, Yao H, Li J, Li S, Xu S (2011) Selenium regulation gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res (in press). doi:10.1007/s12011-011-9166-y
Schara U, Kress W, Bonnemann CG, Breitbach-Faller N, Korenke CG, Schreiber G, Stoetter M, Ferreiro A, von der Hagen M (2008) The phenotype and longterm follow up in 11 patients with juvenile selenoprotein N1 related myopathy. Eur J Paediatr Neurol 12(3):224–230
Small-Howard A, Morozova N, Stoytcheva Z, Forry EP, Mansell JB, Harney JW, Carlson BA, Xu XM, Hatfield DL, Berry MJ (2006) Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol 26(6):2337–2346
Sun B, Wang RH, Li JL, Jiang ZH, Xu SW (2011) Dietary Selenium Affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res (in press). doi:10.1007/s12011-011-8995-z
Tajsharghi H, Darin N, Tulinius M, Oldfors A (2005) Early onset myopathy with a novel mutation in the selenoprotein N gene. Neuromuscul Disord 15(4):299–302
Wang RH, Sun B, Zhang ZW, Li S, Xu SW (2011) Dietary selenium influences pancreatic tissue levels of selenoprotein W in chickens. J Inorg Biochem 105(9):1156–1160
Whanger PD (2000) Selenoprotein W: a review. Cell Mol Life Sci 57(13–14):1846–1852
Whanger PD, Vendeland SC, Beilstein MA (1993) Some biochemical properties of selenoprotein W. In: Anke M, Meissner D, Mills CL (eds) Trace elements in man and animals. Verlag Media Touristik, Gersdor, pp 119–126
Wu XS, Wei CW, Pan CL, Duan Y, Huang KH (2010) Regulation of expression and activity of selenoenzymes by different forms and concentrations of selenium in primary cultured chicken hepatocytes. Br J Nutr 104:1605–1612
Xu XM, Carlson BA, Irons R, Mix H, Zhong N, Gladyshev VN, Hatfield DL (2007a) Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem J 404(1):115–120
Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL (2007b) Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol 5(1):e4
Yu D, Li JL, Zhang JL, Gao XJ, Xu S (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res (in press). doi:10.1007/s12011-011-9062-5
Acknowledgments
This study was supported by the National Natural Science Foundation of China (30871902).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Jl., Li, JL., Huang, Xd. et al. Dietary selenium regulation of transcript abundance of selenoprotein N and selenoprotein W in chicken muscle tissues. Biometals 25, 297–307 (2012). https://doi.org/10.1007/s10534-011-9502-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9502-x