Abstract
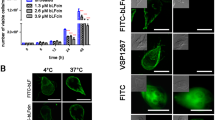
Entamoeba histolytica is a parasitic protozoan that produces amoebiasis, an intestinal disease characterized by ulcerative colitis and dysentery. In some cases, trophozoites can travel to the liver leading to hepatic abscesses and death. Recently, lactoferrin and lactoferricin B have been shown to be amoebicidal in axenic cultures. The aim of this work was to determine whether the lactoferrin-peptides lactoferricin amino acids 17–30, lactoferrampin amino acids 265–284, and lactoferrin chimera which is a fusion product of the two peptides, are capable of producing a microbicidal effect to trophozoites of E. histolytica. We evaluated the killing effect of these peptides in growth kinetics carried out in axenic culture medium to which different concentrations of peptides were added. At 50 μM of peptide concentration, lactoferricin and lactoferrampin had a moderate amoebicidal effect, since a 45–50% of trophozoites remained viable at 24 h culture. However, at 50 μM of the lactoferrin chimera 75% amoeba were killed whereas at 100 μM all cells died. These data indicate that of lactoferrin-peptides mainly the chimera have amoebicidal activity in a time- and concentration-dependent manner. The lactoferrin-peptides might be useful as therapeutic agents against amoebiasis and thereby diminish the use of metronidazole, which is extremely toxic for the host.

Similar content being viewed by others
References
Arnold RR, Russell JE, Champion WJ, Brewer M, Gauthier JJ (1982) Bactericidal activity of human lactoferrin: differentiation from the stasis of iron deprivation. Infect Immun 35:792–799
Baker EM, Baker HM (2009) A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 91:3–10
Bellamy W, Takase M, Wakabayashi H, Kawase K, Tomita M (1992) Antibacterial spectrum of lactoferricin B, a potent bacterial peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol 73:472–479
Bolscher JG, Adao R, Nazmi K, van den Keybus P, van’t Hof W, Nieuw AV, Baston E, Veeman E (2009) Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength that its constituent lactoferricin and lactoferrampin peptides. Biochimie 91:123–132
Brock JH (2002) The physiology of lactoferrin. Biochem Cell Biol 80:1–6
Diamond LS, Harlow DR, Cunnick CC (1978) A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg 72:431–432
Dobias L, Cerna M, Rossner P, Sram R (1994) Genotoxicity and carcinogenicity of metronidazole. Mutat Res 317:177–194
Espinosa-Cantellano M, Martinez-Palomo A (2000) Pathogenesis of intestinal amoebiasis: from molecules to disease. Clin Microbiol Rev 13:318–331
Haney EF, Lau F, Vogel HJ (2007) Solution structure and model membrane interaction of lactoferrampin, an antimicrobial peptide derived from bovine lactoferrin. Biochim Biophys Acta 1768:2355–2364
Haney EF, Nazmi K, Lau F, Bolscher JG, Vogel HJ (2009) Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 91:141–154
Harder R, Greif G, Haberkorn A (2001) Chemotherapeutic approaches to protozoan: Giardia lamblia, Trichomonas and Entamoeba-current level of knowledge and outlook. Parasitol Res 87:785–786
León-Sicairos N, Reyes-López M, Canizalez-Román A, Bermúdez-Cruz RM, Serrano-Luna J, Arroyo R, de la Garza M (2005) Human hololactoferrin: endocytosis and use as an iron source by the parasite Entamoeba histolytica. Microbiology (UK) 151:3859–3871
León-Sicairos N, López-Soto F, Reyes-López M, Godinez Vargas D, Ordaz-Pichardo C, de la Garza M (2006a) Amoebicidal activity of milk, apo-lactoferrin, sIgA and lysozyme. Clin Med Res 4:106–113
León-Sicairos N, Reyes-López M, Ordaz-Pichardo C, de la Garza M (2006b) Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem Cell Biol 84:327–336
León-Sicairos N, Canizalez-Román A, de la Garza M, Reyes-López M, Zazueta-Beltrán J, Nazmi K, Gómez-Gil B, Bolscher JG (2009) Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91:133–140
Lönnerdal B, Iyer S (1995) Lactoferrin molecular structure and biological function. Annu Rev Nutr 15:93–110
Marr AK, Jenssen H, Moniri MR, Hancock RE, Panté N (2009) Bovine lactoferrin and lactoferricin interfere with intracellular trafficking of Herpes simplex virus-1. Biochimie 91:160–164
Masson PL, Heremans JF, Prignot JJ, Wauters G (1966) Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax 21:538–544
Roe FJ (1983) Toxicologic evaluation of metronidazole with particular reference to carcinogenic, mutagenic and teratogenic potential. Surgery 93:158–164
Sánchez-Gómez S, Lamata M, Leiva J, Blondelle SE, Jerala R, Andrä J, Brandenburg K, Lohner K, Moriyón I, Martínez de Tejada G (2008) Comparative analysis of selected methods for the assessment of antimicrobial and membrane-permeabilizing activity: a cse study for lactoferricin derived peptides. BMC Microbiol 8:196–204
Serrano-Luna J, Arzola J, Reyes-López M, de la Garza M (1998) Iron and Entamoeba histolytica HM-1:IMSS. In: Monduzze (ed) IX Proc Int Congr Parasitol. Bologna, Italy, pp 827–830
Tomita M, Wakabayashi H, Shin K, Yamauchi K, Yaeshima T, Iwatsuki K (2009) Twenty-five years of research on bovine lactoferrin applications. Biochimie 91:52–57
Valenti P, Berlutti F, Conte MP, Longhi C, Seganti L (2004) Lactoferrin functions: current status and perspectives. J Clin Gastroenterol 38:S127–S129
Van der Kraan MI, Groenink J, Nazmi K, Veeman EC, Bolscher JG, Nieuw Ameronger AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptide 25:177–183
Van der Kraan MI, Van der Made C, Nazmi K, van’t Hof W, Groenink J, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2005) Effect of amino acid substitutions on the candidacidal activity of LFampin 265–284. Peptide 26:2093–2097
Van der Kraan MI, Nazmi K, van’t Hof W, Amerongen AV, Veerman EC, Bolscher JG (2006) Distinct bactericidal activities of bovino lactoferrin peptides LFampin 268–284 and LFampin 265–284: Asp-Leu-Ile makes a difference. Biochem Cell Biol 84:358–362
Van Snick JL, Masson PL, Heremans JF (1974) The involvement of lactoferrin in the hyposiderhaemia of acute inflammation. J Exp Med 140:1068–1084
Wakabayashi H, Abi S, Okutomi T, Tansho S, Kawase K, Yamaguchi H (1996) Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol Immunol 40:821–825
Wassmann C, Hellberg A, Tannich A, Bruchhaus I (1999) Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem 274(37):26051–26056
Weinberg ED (2003) The therapeutic potential of lactoferrin. Expert Opin Investig Drugs 12:841–851
Wilson ME, Britigan BE (1998) Iron acquisition by parasitic protozoa. Parasitol Today 14:348–353
Wooldridge KG, Williams PH (1993) Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev 12:325–334
Yamauchi K, Tomita M, Giehl TJ, Elison RT III (1993) Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun 61:719–728
Acknowledgments
This work was supported by grant 60102 from Conacyt, Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Soto, F., León-Sicairos, N., Nazmi, K. et al. Microbicidal effect of the lactoferrin peptides Lactoferricin17–30, Lactoferrampin265–284, and Lactoferrin chimera on the parasite Entamoeba histolytica . Biometals 23, 563–568 (2010). https://doi.org/10.1007/s10534-010-9295-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9295-3