Abstract
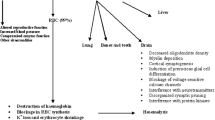
Lead (Pb) toxicity has been a serious concern in industrialized societies because of its association with functional deficits in nervous, haematopoietic and renal systems. Several studies have shown beneficial effects of thiamine on Pb toxicity. It is speculated that Pb chelation by thiamine may be a possible mechanism. However, the exact nature of these interactions remained elusive. In the present study we have characterized the interaction of Pb with thiamine using UV–Vis as well as fluorescence spectroscopic methods and studied the effect of thiamine treatment on blood and tissue Pb levels during simultaneous or post-exposure to Pb in rat model. The spectroscopic studies revealed that Pb interacts with the pyrimidine ring of thiamine, leading to its solubilization at physiological pH. Further, thiamine reduced the Pb levels in blood, kidney and bone during both simultaneous and post-exposure Pb treatment. Interestingly, thiamine appears to prevent the accumulation of Pb in bone during simultaneous treatment. Together these results suggest that pyrimidine ring of thiamine mediates its interaction with Pb, leading to the prevention of its accumulation and/or increased clearance from tissues.





Similar content being viewed by others
References
Anetor JI, Ajose OA, Adebiyi JA, Akingbola TS, Iyanda AA, Ebesunu MO, Babalola OO, Aadeniyi FA (2007) Decreased thiamine and magnesium levels in the potentiation of the neurotoxicity of lead in occupational lead exposure. Biol Trace Elem Res 116:43–51
Ball FM (2006) Vitamins in foods: analysis, bioavailability, and stability. CRC Press, USA, pp 592–595
Bolanos AA, Demizio JP Jr, Vigorita VJ, Bryk E (1996) Lead poisoning from an intra-articular shotgun pellet in the knee treated with arthroscopic extraction and chelation therapy. A case report. J Bone Joint Surg Am 78:422–426
Bosso ST, Enzweiler J (2008) Bioaccessible lead in soils, slag, and mine wastes from an abandoned mining district in Brazil. Environ Geochem Health 30:219–229
Bratton GR, Zmudzki J, Bell MC, Warnock LG (1981) Thiamine effects on lead intoxication and deposition of lead in tissues. Therapeutic potential. Toxicol Appl Pharmaco 59:164–172
Casas JS, Castellano EE, Couce MD, Ellena J, Sanchez A, Sordo J, Taboada C (2006) Zinc(II), cadmium(II) and mercury(II) complexes of the vitamin B1 antagonist oxythiamine. J Inorg Biochem 100:124–132
Dodi K, Gerothanassis IP, Hadjiliadis N, Hadjiliadis N, Bau R, Butler IS, Barrie PJ (1996) Complexesof Zn2+ , Cd2+ , and Hg2+ with 2-(α-Hydroxybenzyl)thiamine monophosphate chloride. Inorg Chem 35:6513–6519
Flora SJS (2002) Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med 12:51–65
Flora SJS, Singh S, Tandon SK (1986) Chelation in metal intoxication XVIII: combined effects of thiamine and calcium disodium versenate on lead toxicity. Life Sci 38:67–71
Flora SJS, Mehta A, Rao PVL, Kannan GM, Bhaskar ASB, Dube SN, Panth BP (2004) Therapeutic potential of monoisoamyl and monomethyl esters of meso 2, 3-dimercaptosuccinic acid in gallium arsenide intoxicated rat. Toxicology 195:127–146
Flora SJS, Flora G, Saxena G (2006) Environmental occurrence, health effects and management of lead poisoning. In: Cascas SB, Sordo J (eds) Lead chemistry, analytical aspects, environmental impacts and health effects. Elsevier Publication, Netherlands, pp 158–228
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128:501–523
Goyer RA (1971) Lead toxicity: a problem in environmental pathology. Am J Pathol 64:167–182
Granick S, Sassa JL, Granick JL, Levere KD, Kappas A (1972) Assays for porphyrin, delta-aminolivulinic acid dehydratase and porphyrinogen synthetase in microliter samples of whole blood: application in metabolic defects involving the heme pathway. Proc Natl Acad Sci USA 69:2381–2385
Horak E, Hopfer SM, Sunderman FW Jr (1981) Spectrophotometric assay for urinary N-Acetyl-β-D-glucosaminidase activity. Clin Chem 27(11):1180–1185
Hu NH, Norifusa T, Aoki K (1999) Interactions of metal ions with the intermediate of thiamine catalysis: Crystal structures of Cd(II), Hg(II) and Pt(II) complexes of 2-([alpha]-hydroxybenzyl)thiamine. Polyhedron 18:2987–2994
Hu NH, Aoki K, Adeyemo AO, Williams GN (2001) Metal ion and anion coordination in the thiamine—[PtII(NO2)4]2—system. Structures of a metal complex, Pt(thiamine)(NO2)3, and two salts, (H-thiamine)[Pt(NO2)4]·2H2O and (thiamine monophosphate)2[Pt(NO2)4]·2H2O. Inorg Chimica Acta 325:9–19
Laurence, Bacharah AL (1964) Evaluation of drug activities: pharmacometrics, vol 2. Academic Press, New York, pp 615–619
Louis-Ferdinand RT, Glass L, Sharp R, Beuthin FC (1982) Altered lead distribution in thiamine-treated rodents. Toxicologist 2:82 Abs
Malandrinos G, Louloudi M, Mitsopoulou CA, Butler IS, Bau R, Hadjiliadis N (1998) On the mechanism of action of thiamin enzymes, crystal structure of 2- (α-hydroxyethyl)thiamin pyrophosphate (HETPP). Complexesof HETPP with zinc(II) and cadmium(II). J Biol Inorg Chem 3:437–448
Malandrinos G, Louloudi M, Koukkou AI, Sovago I, Drainas C, Hadjiliadis N (2000) Zinc(II) and cadmium(II) metal complexes of thiamine pyrophosphate and 2-(alpha-hydroxyethyl)thiamine pyrophosphate: models for activation of pyruvate decarboxylase. J Biol Inorg Chem 5:218–226
Malandrinos G, Louloud M, Hadjiliadis N (2006) Thiamine models and perspectives on the mechanism of action of thiamine-dependent enzymes. Chem Soc Rev 35:684–692
Papanikolaou NC, Hadzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM (2005) Lead toxicity update: a brief review. Med Sci Monit 11:RA329–RA336
Preuss HG (1993) A review of persistent, low-grade lead challenge: neurological and cardiovascular consequences. J Am Coll Nutr 12:246–254
Pullakhandam R, Nair MK, Kasula S, Kilari S, Thippande TG (2008) Ferric reductase activity of low molecular weight human milk fraction is associated with enhanced iron solubility and uptake in CaCo-2 cells. Biochem Biophys Res Commun 374:369–372
Ryan MA, Ingle JD Jr (1980) Fluorometric reaction rate method for the determination of thiamine. Anal Chem 52:2177–2184
Sasser LB, Hall GG, Bratton GR, Zmudzki J (1984) Absorption and tissue distribution of lead in thiamine-replete and thiamine-deficient rats. J Nutr 114:1816–1825
Saxena G, Joshi U, Flora SJS (2005) Monoesters of meso 2, 3-dimercaptosuccinic acid in lead mobilization and recovery of lead induced tissue oxidative injury in rats. Toxicology 214:39–56
Segura-Carretero A, Costa-Fernández JM, Pereiro R, Sanz-Medel A (1999) Low-level mercury determination with thiamine by fluorescence optosensing. Talanta 49:907–913
Stamatis A, Malandrinos G, Louloudi M, Hadjiliadis N (2007) New perspectives on thiamine catalysis: from enzymic to biomimetic catalysis. Bioinorg Chem Appl 2007:23286–23293
Subramanian KS, Meranger JC (1981) A rapid electrothermal atomic absorption spectrophotometric method for cadmium and lead in human whole blood. Clin Chem 27:1866–1871
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev 49:529–554
Yeazer DW, Cholar KJ, Handerson EW (1971) Determination of lead in biological and related malnutrition by AAS. Environ Sci Techno 5:1020–1022
Acknowledgments
We thank Dr. B. Sesikeran, Director, National Institute of Nutrition, Hyderabad for his interest in the studies. We also thank Mr. K. Sreenivasulu for his critical review and suggestions. SYR is supported by a Senior Research Fellowship from NIN-Indian Council of Medical Research (ICMR), Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, S.Y., Pullakhandam, R. & Dinesh Kumar, B. Thiamine reduces tissue lead levels in rats: mechanism of interaction. Biometals 23, 247–253 (2010). https://doi.org/10.1007/s10534-009-9282-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9282-8