Abstract
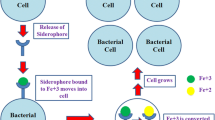
The Rhizobia comprise one of the most important groups of beneficial bacteria, which form nodules on the roots (rarely on the stems) of leguminous plants. They live within the nodules and reduce atmospheric nitrogen to ammonia, which is further assimilated by plants into required nitrogenous compounds. The Rhizobia in return obtain nutrition from the plant. Rhizobia are free-living soil bacteria and have to compete with other microorganisms for the limited available iron in the rhizosphere. In order to acquire iron Rhizobia have been shown to express siderophore-mediated iron transport systems. Rhizobium leguminosarum IARI 917 was investigated for its ability to produce siderophore. It was found to produce a dihydroxamate type siderophore under iron restricted conditions. The siderophore was purified and chemically characterized. The ESMS, MS/MS and NMR analysis indicate the dihydroxamate siderophore to be ‘schizokinen’, a siderophore reported to be produced by Bacillus megaterium that shares a similar structure to ‘rhizobactin 1021’ produced by Sinorhizobium meliloti 1021. This is the first report of production of schizokinen by a strain of R. leguminosarum, therefore it was carefully investigated to confirm that it is indeed ‘schizokinen’ and not a degradation product of ‘rhizobactin 1021’. Since ferric–siderophore complexes are transported across the outer membrane (OM) into the periplasm via an OM receptor protein, R. leguminosarum IARI 917 was investigated for the presence of an OM receptor for ‘ferric–schizokinen’. SDS PAGE analysis of whole cell pellet and extracted OM fractions indicate the presence of a possible iron-repressible OM receptor protein with the molecular weight (MW) of approximately 74 kDa.
Similar content being viewed by others
References
Akers HA (1983) Isolation of the siderophore schizokinen from soil of rice fields. Appl Environ Microbiol 45: 1704–1706
Archibald F (1983) Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol Lett 19: 29–32
Arnow LE (1937) Colorimetric determination of the components of 3,4-dihydroxyphenylalanine–tyrosine mixtures. J Biol Chem 118: 531–537
Atkin CL, Neilands JB, Phaff HJ (1970) Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol 103: 722–733
Braun V, Hantke K, Koster W (1998) Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst 35: 67–145
Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. (1999) Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol 6: 56–63
Byers BR, Powell MV, Lankford CE. (1967) Iron-chelating hydroxamic acids (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol 93: 286–294
Carson KC, Meyer J, Dilworth MJ. (2000) Hydroxamate siderophores of root nodule bacteria. Soil Biol Biochem 32: 11–21
Chakraborty R, Lemke E, Cao Z, Klebba PE, van der Helm D. (2003) Identification and mutational studies of conserved amino acids in the outer membrane receptor protein, FepA, which affect transport but not binding of ferric-enterobactin in Escherichia coli. Biometals 16: 507–518
Cobessi D, Celia H, Folschweiller N, Schalk IJ, Abdallah MA, Pattus F. (2005) The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6 resolution. J Mol Biol 347: 121–134
Cuiv PO, Lynch D, O’Connell M. (2004) Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J Bacteriol 186: 2996–3005
de Lorenzo V, Bindereif A, Paw BH, Neilands JB. (1986) Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol 165: 570–578
de Lorenzo V, Neilands JB. (1986) Characterization of iucA and iucC genes of the aerobactin system of plasmid ColV-K30 in Escherichia coli. J Bacteriol 167: 350–355
Diarra M, Lavoie MC, Jacques M, Darwish I, Dolence EK, Dolence JA, Ghosh A, Ghosh M, Miller MJ, Malouin F. (1996) Species selectivity of new siderophore-drug conjugates that use specific iron uptake for entry into bacteria. Antimicrob Agents Chemother 40: 2610–2617
Dilworth MJ, Carson KC, Giles RGF, Byrne LT, Glenn AR. (1998) Rhizobium leguminosarum bv. viciae produces a novel cyclic trihydroxamate siderophore, vicibactin. Microbiology 144: 781–791
Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. (1998) Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282: 2215–2220
Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J. (2002) Structural basis of gating by the outer membrane transporter FecA. Science 295: 1658–1659
Gabelica V, De Pauw E. (2005) Internal energy and fragmentation of ions produced in electrospray sources. Mass Spectrom Rev 24: 566–587
Gibson F, McGrath DI. (1969) The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta 192: 175–184
Gledhill M. (2001) Electrospray ionization-mass spectrometry of hydroxamate siderophores. Analyst 126: 1359–1362
Guiseppi JD, Fridovich I. (1982) Oxygen toxicity in Streptococcus sanguis. J Biol Chem 257: 4046–4051
Hu X, Boyer GL. (1995) Isolation and characterization of the siderophore N-deoxyschizokinen from Bacillus megaterium ATCC 19213. Biometals 8: 357–364
Jadhav R, Desai AJ. (1996) Effect of nutritional and environmental conditions on siderophore production by Rhizobium GN1 (peanut isolate). Indian J Exp Biol 34: 436–439
Jalal MAF, van der Helm D. (1991) Isolation and spectroscopic identification of fungal siderophores. In: Winkelmann G (eds), Handbook of Microbial Iron Chelates. Boca Raton FL, CRC Press, pp. 235–269
Johnston AWB. (2004) Mechanisms and regulation of iron uptake in the Rhizobia. In: Crosa JH, Mey AR, Payne SM (eds). Iron Transport in Bacteria. Washington DC, ASM Press, pp. 469–488
Kilz S, Lenz C, Fuchs R, Budzikiewicz H. (1999) A fast screening method for the identification of siderophores from fluorescent Pseudomonas spp. by liquid chromatography/electrospray mass spectrometry. J Mass Spectrom 34: 281–290
Kneen B, Larue TA. (1983) Congo red absorption by Rhizobium leguminosarum. Appl Environ Microbiol 45: 340–342
Lee B, Miller MJ. (1983) Natural ferric ionophores: total synthesis of schizokinen, schizokinen A, and arthrobactin. J Org Chem 48: 24–31
Litwin CM, Calderwood SB. (1993) Role of iron in the regulation of virulence genes. Clin Microbiol Rev 6: 137–149
Lynch D, O’Brien J, Welch T, Crosa JH, O’Connell M. (2001) Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J Bacteriol 183: 2576–2585
Martinez JS, Zhang GP, Holt PD, Jung HT, Carrano CJ, Haygood MG, Butler A. (2000) Self-assembling amphiphilic siderophores from marine bacteria. Science 287: 1245–1247
McLafferty FW, Tureček F. (1993) Interpretation of mass spectra, Fourth Edition. University Science Books, Mill Valley CA
Mullis KB, Pollack JR, Neilands JB. (1971) Structure of schizokinen, an iron-transport compound from Bacillus megaterium. Biochemistry 10: 4894–4898
Neilands J. (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 45: 26723–26726
O’Brien IG, Gibson F. (1970) The structure of enterochelin and related 2,3-dihydroxy-N-benzoyl-serine conjugates from Escherichia coli. Biochim Biophys Acta 215: 393–402
Patel H, Chakraborty RN, Desai SB. (1988) Isolation and partial characterization of phenolate siderophore from Rhizobium leguminosarum IARI 102. FEMS Microbiol Lett (UK) 56: 131
Persmark M, Pittman P, Buyer JS, Schwyn B, Gill PR, Neilands JB. (1993) Isolation and structure of rhizobactin 1021, a siderophore, from the alfalfa symbiont Rhizobium meliloti 1021. J Am Chem Soc 115: 3950–3956
Postle K. (1993) TonB protein and energy transduction between membranes. J Bioenerg Biomembr 25: 595–601
Raymond KN, Dertz EA, Kim SS. (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci 100: 3584–3588
Raymond K, Dertz EA. (2004) Biochemical and physical properties of siderophores. In: Crosa J, Mey AR, Payne SM, (eds), Iron Transport in Bacteria. Washington DC, ASM Press, pp. 3–17
Reigh G, O’Connell M. (1993) Siderophore-mediated iron transport correlates with the presence of specific iron-regulated proteins in the outer membrane of Rhizobium meliloti. J Bacteriol 175: 94–102
Schwyn B, Neilands JB. (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160: 47–56
Smith MJ, Shoolery JN, Schwyn B, Holden I, Neilands JB. (1985) Rhizobactin, a structurally novel siderophore from Rhizobium meliloti. J Am Chem Soc 107: 1739–1743
Spasojevic I, Boukhalfa H, Stevens RD, Crumbliss AL. (2001) Aqueous solution speciation of Fe(III) complexes with dihydroxamate siderophores alcaligin and rhodotorulic acid and synthetic analogues using electrospray ionization mass spectrometry. Inorg Chem 40: 49–58
Stevens JB, Carter RA, Hussain H, Carson KC, Dilworth MJ, Johnston AWB. (1999) The fhu genes of Rhizobium leguminosarum, specifying siderophore uptake proteins: fhuDCB are adjacent to a pseudogene version of fhuA. Microbiology 145: 593–601
Storey EP. 2005 Isolation, purification and chemical characterization of the dihydroxamate-type siderophore, “schizokinen” produced by Rhizobium leguminosarum IARI 917. MS Thesis: East Tennessee State University, Johnson City, TN.
van der Helm D. (2004) Structure of outer membrane receptor proteins. In: Crosa JH, Mey AR, Payne SM, (eds). Iron Transport in Bacteria. Washington DC, ASM Press, pp. 51–65
Vellore J. 2001 Iron acquisition in Rhodococcis erythropolis strain IGTS8: isolation of a non-siderophore producing mutant. MS Thesis: East Tennessee State University, Johnson City, TN.
Visca P, Leoni L, Wilson MJ, Lamont IL. (2002) Iron transport and regulation, cell signaling and genomics: lessons from Escherichia coli and Pseudomonas. Mol Microbiol 45: 1177–1190
Wandersman C, Delepelaire P. (2004) Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58: 611–647
Waring WS, Workman CH. (1942) Growth of bacteria in an iron-free medium. Arch Biochem 1: 303–310
Yeoman K, Wisniewski-Dye F, Timony C, Stevens JB, deLuca NG, Johnston AWB. (2000) Analysis of R. leguminosarum siderophore-uptake gene fhuA: differential expression in free living bacteria and nitrogen-fixing bacteroids and distribution of a fhuA pseudogene in different strains. Microbiology 146: 829–837
Acknowledgements
We thank Bert Lampson and Michael Gallagher for critical reading of the manuscript, Ralph Coffman and Brianne Clark for technical support and Dr. H.N. Patel for sending the culture of R. leguminosarum IARI 917. This study was supported by Department of Health Sciences, ETSU, analytical facilities of Eastman Chemical Company, Kingsport, TN and grants, GM 069367 (RC) from NIH and RDC 03-008M (RC) from ETSU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Storey, E., Boghozian, R., Little, J.L. et al. Characterization of ‘Schizokinen’; a dihydroxamate-type siderophore produced by Rhizobium leguminosarum IARI 917. Biometals 19, 637–649 (2006). https://doi.org/10.1007/s10534-006-9001-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9001-7