Abstract
The regulatory roles of temperature, eutrophication and oxygen availability on benthic nitrogen (N) cycling and the stoichiometry of regenerated nitrogen and phosphorus (P) were explored along a Baltic Sea estuary affected by treated sewage discharge. Rates of sediment denitrification, anammox, dissimilatory nitrate reduction to ammonium (DNRA), nutrient exchange, oxygen (O2) uptake and penetration were measured seasonally. Sediments not affected by the nutrient plume released by the sewage treatment plant (STP) showed a strong seasonality in rates of O2 uptake and coupled nitrification–denitrification, with anammox never accounting for more than 20 % of the total dinitrogen (N2) production. N cycling in sediments close to the STP was highly dependent on oxygen availability, which masked temperature-related effects. These sediments switched from low N loss and high ammonium (NH4 +) efflux under hypoxic conditions in the fall, to a major N loss system in the winter when the sediment surface was oxidized. In the fall DNRA outcompeted denitrification as the main nitrate (NO3 −) reduction pathway, resulting in N recycling and potential spreading of eutrophication. A comparison with historical records of nutrient discharge and denitrification indicated that the total N loss in the estuary has been tightly coupled to the total amount of nutrient discharge from the STP. Changes in dissolved inorganic nitrogen (DIN) released from the STP agreed well with variations in sedimentary N2 removal. This indicates that denitrification and anammox efficiently counterbalance N loading in the estuary across the range of historical and present-day anthropogenic nutrient discharge. Overall low N/P ratios of the regenerated nutrient fluxes impose strong N limitation for the pelagic system and generate a high potential for nuisance cyanobacterial blooms.
Similar content being viewed by others
Introduction
The Baltic Sea has a long history of eutrophication and water anoxia (Zillén et al. 2008; Conley et al. 2009). The spreading of hypoxia and anoxia in the bottom waters since the 1960s (Elmgren 2001; Conley et al. 2009) is generally thought to be responsible for significant N loss due to enhanced denitrification in the water column (Rönner 1985; Vahtera et al. 2007). While an N deficit in the Baltic has been identified based on budget calculations (Conley et al. 2009), coupled physical biogeochemical models (Meier et al. 2012), and gas inventories (Löffler et al. 2011), there have been few direct rate measurements in sediments and the water column (Deutsch et al. 2010; Jäntti et al. 2011; Hietanen et al. 2012; Dalsgaard et al. 2013). The regional and spatial coverage of benthic flux data for the Baltic is low and most of the existing studies have not investigated the key regulators of benthic N cycling such as temperature, oxygen availability and demand, presence/absence of macrofauna, sedimentary organic matter content, or variable nutrient loading from human activities. Furthermore, for some of the most highly impacted areas of the Baltic Sea the published rates of N2 production refer to a single campaign and do not have a temporal resolution that is high enough to catch the seasonal variability or the steep horizontal and vertical gradients of potentially regulating factors (Hietanen 2007; Deutsch et al. 2010; Jäntti and Hietanen 2012).
Even though major nutrient reduction strategies have been agreed to for the Baltic Sea catchment area, hypoxia has further expanded over the past 10 years and now affects both central basins and the coastal zone (Conley et al. 2009; Conley et al. 2011). Recently it has been suggested that the reduction of nutrient levels alone may not be able to neutralize or reverse the impact of eutrophication (Duarte et al. 2009). This points to the importance of internal dynamics for sustaining long-term eutrophication and emphasizes the need for temporally and spatially resolved benthic studies focusing on respiration and nutrient regeneration. Disappearance/decrease of the oxic sediment surface layer following anoxia/hypoxia makes the path of NO3 − to the anoxic horizon thinner (Jensen et al. 1994; Rysgaard et al. 1994). This can either stimulate denitrification if NO3 − is abundant in the bottom water, or inhibit nitrification, which is generally thought to be the major NO3 − source within sediments, and can therefore limit denitrification (Jensen et al. 1994). Furthermore, excess accumulation of organic matter with low C/N ratio and the occurrence of free hydrogen sulfide can favor the dissimilatory reduction of nitrate to ammonium (DNRA)—a process that leads to N recycling instead of N removal (Christensen et al. 2000; An and Gardner 2002). The wide significance of DNRA in estuarine and coastal sediments has been recognized only very recently (Gardner et al. 2006; Dong et al. 2011), and it has been suggested that its importance was largely underestimated in previous budgets (Burgin and Hamilton 2007). Finally, anammox (the anaerobic oxidation of ammonium coupled to nitrite reduction) was demonstrated to play a role together with denitrification in the removal of fixed N2 in coastal zones and estuaries, even though anammox accounts for ≤30 % of the N2 production in such ecosystems (Dalsgaard et al. 2005; Hulth et al. 2005). However, the relevance of anammox in systems affected by variable degrees of eutrophication and hypoxia is still debated. Recent experiments in the North Sea revealed that anammox rates did not change during exposure to short-term hypoxia (Neubacher et al. 2011), whereas prolonged hypoxia significantly stimulated anammox activity (Neubacher et al. 2013).
Under anoxic conditions, inorganic P is efficiently regenerated, in particular in iron-rich sediments (Sundby et al. 1992). It is not clear how bottom water oxygen concentrations and organic matter content and composition affect benthic microbial N transformation pathways and alter the stoichiometry of regenerated N and P in eutrophic seas. In particular the proportion of recycled N as NH4 + versus the amount of lost N via denitrification or anammox need to be better quantified and related to the regenerated P pool (Vahtera et al. 2007; Jäntti and Hietanen 2012).
This study presents a comprehensive set of seasonal measurements carried out in an estuary on the western border of the Baltic Sea, which has a 40 year-long record of anthropogenic organic matter and nutrient loading. Sedimentary O2 consumption, NO3 − reduction processes, inorganic N and P regeneration and their regulating factors were investigated in intact sediments, collected in a grid of stations along a well-established eutrophication gradient. To our knowledge, this is one of the few studies where an array of benthic N processes, the stoichiometry of N and P regeneration and their porewater profiles, C and N sedimentary stable isotope composition, diffusive and total O2 uptake were simultaneously measured on a seasonal basis along an anthropogenically impacted estuary. Finally, a budget of N and P recycled in the estuary is proposed and compared to a previous scenario when the STP discharged three times more nutrients than nowadays.
Materials and methods
Site description
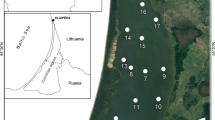
Himmerfjärden (Fig. 1) is an estuary with a surface area of 174 km2 and a north to south salinity gradient increasing from 5.5 in the inner part to 7.0 at the opening to the Baltic. Himmerfjärden is morphologically characterized by four basins, divided by a number of sills. Water sources to the estuary derive from: (1) moderate land run-off, (2) outflow from Lake Mälaren in the northernmost part, (3) discharge of the sewage treatment plant (STP) (Engqvist and Omstedt 1992). The STP, built in the early 1970s, treats sewage water from 280,000 inhabitants and its inorganic effluent is discharged mainly in the form of DIN and DIP to the inner basins (Savage et al. 2004). The estuary undergoes thermohaline stratification during late summer and autumn, especially in the inner part, which experiences regular seasonal hypoxia in the bottom water. Hypoxia has also been reported occasionally for the outer basins of the estuary when winds are weak and circulation is inhibited (Elmgren and Larsson 1997).
Bottom water and sediment samples were taken from three stations along Himmerfjärden (H6, H4 and H2), and from one “control” station located outside the morphological boundaries of the estuary, with the same salinity of the open Baltic proper (B1) (Fig. 1). Samples were collected in May, June, August, October 2011 and January 2012 (Table 1). Stations B1 and H2 have soft muddy sediment, while the sediment at H4 is characterized by muddy clay and fine sand. Station H6 is located close to the STP in the inner part of the bay and the sediment at this station is soft, laminated black mud. Sediment accumulation rates range from 0.98 cm year−1 in the innermost part of the estuary to 0.77 cm year−1 in the outermost part (Thang et al. 2013).
Sampling activities
Temperature, salinity and dissolved oxygen in the water column were measured with a CTD system equipped with a fast-responding Rinko oxygen optode (Sea & Sun Technology), which was field-calibrated before each deployment. Bottom water was collected with a 5 L Niskin bottle. Sediment was retrieved in acrylic core liners (i.d. 9 cm, height 60 cm, n = 8–10 for each station) using a multicorer (K.U.M. Umwelt- und Meerestechnik Kiel). Intact cores with undisturbed sediment surface were subsampled onboard in smaller acrylic liners (i.d. 3.6 cm, height 25 cm). To avoid sediment compaction during subsampling liners with 2-mm thick walls and beveled ends were employed. The sediment height in the subsampled cores was approximately 12 cm. All the sediment cores were subsequently stored in boxes submersed in cooled bottom water from the same station and transported within 1 h after their collection to the Stockholm University Marine Field Station on Askö, where they were maintained in the dark in a cold room and constantly stirred with magnetic bars at in situ temperature.
Sediment characteristics and profiles
From each station an intact sediment core of 9 cm i.d. was sliced at intervals of 0.5 cm down to 2 cm depth for the analysis of the concentration and stable isotope composition of organic carbon and N. Samples were maintained frozen at −80 °C. The sediments were further sliced to 10 cm depth at 1-cm intervals to determine their water content and porosity. The water content was calculated by measuring the wet and dry weight of 5 mL sediment after drying at 105 °C.
A replicate core of the same size was used for nutrient porewater profiles. The core was sectioned into slices of 3 mm for the top 2 cm, then further in intervals of 5 mm to 5 cm depth, and in 10 mm intervals to 10 cm depth. In May 2011 the profiles were measured to a depth of 20 cm. The sediment slices were centrifuged and the supernatant was immediately filtered through Whatman GF/F filters and frozen at −80 °C for later analysis of dissolved inorganic nitrogen (DIN) and dissolved inorganic phosphorus (DIP). Before slicing, the overlying water was sampled with a plastic syringe, filtered and frozen in order to determine nutrient concentrations in the bottom water (Table 1).
At each station three to five O2 microprofiles were measured in at least two 3.6 cm i.d. intact cores using Clark-type oxygen microelectrodes (OX-50, Unisense) mounted on a manual micromanipulator (MM33, Unisense). The vertical resolution was 50 μm. Measurements were performed in the dark at in situ temperature. In order to have sufficient water stirring during measurements an overlying water column of ~2 cm was left in the sediment core and aerated by a flow of atmospheric air. When in situ O2 concentration was far from air saturation the stirring was provided by bubbling the overlying water with a mixture of N2 and air designed to keep oxygen concentrations low. However, due to the small volume of water left in the sediment cores, in several cases it was difficult to reproduce accurately the in situ O2 conditions (see below).
The diffusive flux of PO4 3− and NH4 + (J) was calculated from the Fick’s first law of diffusion:
where φ is sediment porosity, Ds is molecular diffusivity in sediment, and ∂C/∂x is the concentration gradient, assuming steady state. Ds was calculated according to Iversen and Jørgensen (1993):
The diffusion coefficient in free water (D0) was calculated according to Schulz (2006). Sediment oxygen consumption as a function of depth was calculated using the numerical procedure for interpretation of O2 microprofiles, PROFILE (Berg et al. 1998), and from the mean profiles the diffusive oxygen uptake (DOU) was calculated. Equation 2 was used as expression for Ds, while for boundary conditions (BC) the concentration at the bottom and the flux at the bottom of the profile were selected (Berg et al. 1998). We did not consider the O2 microprofiles in the DOU calculation when the difference between the in situ O2 value and the value at which the microprofiles were carried out was >20 %.
Sediment core incubations
21–24 cores with an inner diameter of 3.6 cm were pre-incubated uncapped in a 40 L incubation tank filled with in situ bottom water. The overlying water in the cores was stirred with a magnetic bar driven by an external magnet at 60 rpm. The oxygen concentration in the water tank was adjusted to the bottom water oxygen concentration by bubbling with the appropriate mixture of N2 and air.
On the day after sediment collection, a minimum of five cores from each station were closed with caps while stirring (60 rpm) in order to determine net fluxes of dissolved O2, NH4 +, NO3 −, NO2 −, and total inorganic PO4 3−. The incubation time was set after a preliminary evaluation of the sediment oxygen consumption rates, in order to avoid O2 concentrations decreasing below 20 % of the initial value (Dalsgaard et al. 2000). The sediment cores were incubated for a period of 6–12 h. At the beginning and at the end of the incubation water samples were taken from the incubation tank (n = 4) and from each core, respectively. The water samples were immediately filtered through GF/F Whatman filters and frozen for later determination of nutrient concentrations. The concentration of dissolved oxygen was measured prior and after the incubation in the water overlying the sediment with an oxygen mini-electrode (OX-500, Unisense). The oxygen mini-electrode was calibrated prior to each measurement according to the manufacturer’s recommendation.
Simultaneously, a minimum of 16 cores from each sampling site were incubated to determine the rates of denitrification, anammox and DNRA, according to the revised-isotope pairing technique (r-IPT) (Risgaard-Petersen et al. 2003; Bonaglia et al. 2013). In brief, Na15NO3 (99 % atom, 10 mmol L−1 stock solution) was added to each core in order to reach a final concentration of 25, 50, 100 and 150 μmol L−1 15NO3 − (n = 4–5 for each concentration). Before and after the addition of 15NO3 − water samples were collected from each core, filtered and frozen for later NO3 − analysis in order to calculate the ratio between unlabeled and labeled NO3 −. The incubation started after a lag time, which was necessary to establish a linear production of 15N-N2 within the sediment (Dalsgaard et al. 2000). At the beginning of the incubation the cores were capped with rubber stoppers so that no air bubbles formed and the water was mixed by externally driven magnetic bars. One core of each concentration series was sampled right after the lag time passed, in order to evaluate the background concentration of 15N-N2. The incubation lasted between 6 and 12 h, during which time O2 concentration did not decrease by more than 20 % of the initial value. The incubation was terminated by mixing the water phase with the upper 7–8 cm of sediment, coinciding with the bioturbated part of the sediment. Slurry samples were collected in 7.7 mL Exetainer vials (Labco Scientific, UK), and 100 μL of a 7 mol L−1 ZnCl2 solution was added to stop bacterial activity. Subsequently the samples were analyzed for the 29N2/28N2 and 30N2/28N2 ratios, as described below. An additional sample of the slurry was withdrawn from each core, treated with 2 mol L−1 KCl, centrifuged, filtered, and immediately frozen at −80 °C to determine the 15NH4 + fraction for calculation of DNRA rates. A summary of the incubations and the substrates added is given in Table 2.
Slurry incubations
Potential nitrification rates (PNRs) were measured in oxic slurries enriched with NH4 +. Briefly, 2 mL of fresh sediment were collected from the surface sediment layer (~1 cm) and suspended in 40 mL of GF/F-filtered in situ water, amended with NH4 + to a final concentration of 100 μmol L−1 NH4 +. The slurries (n = 3 for each station) were mixed on a shaker table in the dark and at in situ temperature. After a preincubation period of 15 min the slurries were subsampled, centrifuged, and the supernatant was filtered and frozen for later analysis of initial NH4 +, NO3 − and NO2 − concentrations (DIN). The slurries were incubated for up to 12 h and thereafter sampled again for the determination of the final DIN concentrations.
The anammox contribution to total N2 production in sediments was evaluated by means of anoxic slurry incubations performed in 7.7 mL Exetainer vials (Labco Scientific). 2 mL of fresh sediment were collected from the topmost 1.5 cm and transferred to each Exetainer, which was subsequently filled with anoxic bottom water. The slurries (n = 20 for each station) were preincubated overnight, so that residual O2 and NO3 − were used up. Sediment and water were continuously mixed by placing the vials on a rotating stirrer, and a glass bead was added in each vial. After preincubation, combinations of 14NH4 +, 14NO3 −, 15NH4 + and 15NO3 − (99 % atom from 10 mmol L−1 stock solutions) were added to the anoxic slurries (final concentration 150 μmol L−1). There were four treatments: (1) addition of 15NH4 + and (2) addition of 15NH4 + + 14NO3 − to test for the presence of anammox (detected by the production of 29N2) and to test if all of the pre-existing 14NO3 − was consumed in the preincubation, (3) addition of 15NO3 −, and (4) addition of 15NO3 − + 14NH4 + to calculate the relative contribution of anammox to the total N2 production using the equations reported in Thamdrup and Dalsgaard (2002). Duplicate samples were killed directly after the addition of substrates by injecting 100 μL of 7 mol L−1 ZnCl2 to each Exetainer. The incubation was terminated after 12 h. No additional time points were taken because earlier experiments showed that the increase in total N2 production over 24 h of incubation was linear (Bonaglia, unpubl. data).
Laboratory analysis and calculations
Samples for concentration and stable isotope composition of organic carbon and N were treated with 2 N HCl to remove inorganic carbon. Subsequently, samples were freeze-dried, homogenized, and weighed into silver (C) and tin (N) capsules. The analyses were performed with an elemental analyzer (Carlo Erba NC2500) coupled to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Scientific). Isotopic compositions are reported using the conventional delta notation, which reports the isotopic composition of a sample as the ‰ deviation of a sample relative to atmospheric N2 (δ15N) and relative to PDB (δ13C). IAEA reference materials N1, N2, and NO3 were used for δ15N and CO-1, CO-8, NBS18, and NBS19 for δ13C. Peptone was used as internal lab standard. Based on the analyses of these standards the analytical precision was ± 0.2 ‰.
DIN species (NH4 +, NO3 −, NO2 −) and PO4 3− were determined on a segmented flow autoanalyzer system (ALPKEM, Flow Solution IV). Precision was ± 0.036 μmol L−1 for NH4 +, ± 0.021 μmol L−1 for NO3 −, ± 0.014 μmol L−1 for NO2 −, and ± 0.016 μmol L−1 for PO4 3−. Net fluxes of solutes were calculated according to Bonaglia et al. (2013). Positive values represent effluxes while negative values represent sediment uptake.
The abundance and concentration of 29N2, and 30N2 in the denitrification and anammox experiments were determined by gas chromatography-isotope ratio mass spectrometry modifying a setup described in Holtappels et al. (2011). Samples were injected on-column on a 2 m stainless steel packed Porapak Q column (Supelco). Water was trapped by a liquid nitrogen trap and O2 was reduced by passing the gas through a copper column heated to 650 °C. Samples were introduced to the mass spectrometer through an open split interface (Conflo IV, Thermo Scientific) and analyzed on a Delta V Advantage (Thermo Scientific). 15 μL of air were injected after every 5th sample as standard. Rates of anammox and denitrification were calculated following the procedures described by Thamdrup and Dalsgaard (2002) and Risgaard-Petersen et al. (2003).
In order to measure the isotopic composition of the NH4 + pool, an aliquot of the slurry extract was analyzed by the phenol hypochlorite-indophenol blue method of Solorzano (1969), while another aliquot was transferred to 7.7 mL Exetainers, degassed with helium and treated with a hypobromite-iodine solution to oxidize NH4 + to N2 (Risgaard-Petersen et al. 1995). The conversion was tested with standard solutions of NH4 + and was always >80 %. The isotopic composition of the N2 pool, which reflects the composition of the NH4 + pool, was determined using the same procedure as described for denitrification and anammox samples (GC-IRMS).
Anammox presence was tested with anoxic slurries amended with 15NH4 + (Thamdrup and Dalsgaard 2002), and with cores incubated at different 15NO3 − concentrations (Risgaard-Petersen et al. 2003). When anammox was not detected production of N2 (=denitrification) was estimated following the isotope pairing technique (IPT) by Nielsen (1992), otherwise N2 production (=denitrification + anammox) was likewise calculated referring to the revised-IPT (r-IPT) described in Risgaard-Petersen et al. (2003). In the presence of anammox two different methods were used to calculate denitrification and anammox rates, corresponding to method 1 and method 2 described in Trimmer et al. (2006). Briefly, both methods express the total N2 production (p 14) as a function of r 14, the ratio between 14NO3 − and 15NO3 − in the zone where NO3 − reduction occurs:
where p 29N2 and p 30N2 are the production rates of 29N2 and 30N2, respectively. The parameter r 14 can be estimated from:
where ra is the contribution of anammox to N2 production measured through the procedure described by Thamdrup and Dalsgaard (2002) and R 29 is the ratio between p 29N2 and p 30N2. Thus with method 1 N2 production (p 14) was calculated by combining Eqs. 3 and 4. Method 2 expresses r 14 from the production rates of 29N2 and 30N2 measured in sediment cores incubated at two different 15NO3 − concentrations:
where p 29N (a)2 and p 30N (a)2 refer to the production rates of 29N2 and 30N2 in the incubation at lower 15NO3 − concentration, whereas p 29N (b)2 and p 30N (b)2 refer to the production rates of 29N2 and 30N2 in the incubation at higher 15NO3 − concentration. The parameter V (the ratio between concentrations of 15NO3 − in the overlying water from two incubations at different amendments of 15NO3 −) was obtained from:
With method 2 we calculated r 14 directly from the production rates of 29N2 and 30N2 measured in incubations at four different 15NO3 − concentrations. Values of V and r 14 were calculated for pairwise combinations of two different 15NO3 − amendments using Eqs. 5 and 6. Anammox rate (aao 14) was calculated in both methods according to the formula:
Genuine denitrification rate (den 14) was calculated from the equation:
The total production of N2 was split between production dependent on NO3 − diffusing into the sediment from the bottom water (p 14 w) and production dependent on sedimentary nitrification (p 14 n) expressed as:
where r 14 w is the ratio between the concentrations of 14NO3 − and 15NO3 − in the water column.
Total DNRA rates were calculated modifying the equation described in Risgaard-Petersen and Rysgaard (1995), and were estimated from the production of 15NH4 + (p 15NH4 +) multiplied by r 14.
In order to present an annual budget for the N cycling processes nitrification and ammonification were estimated from nutrient and oxygen fluxes. Nitrification rates were calculated by the sum of NO3 − efflux and p 14 n, assuming absence of benthic microalgae (Rysgaard et al. 2004). Ammonification has to be considered as a potential ammonification rate and was calculated dividing the TOU by the C/N ratio, assuming a CO2/O2 ratio of 1.2 (Dalsgaard 2003). Denitrification efficiency (DE), sensu Eyre and Ferguson (2009), was calculated as the ratio between the DIN flux and the N2 production via denitrification/anammox.
Statistical analyses
Statistical tests were performed in order to detect differences in solute fluxes and process rates between stations. Homogeneity of variance of the dataset was checked using Cochran’s test. Since the variance was generally found to be heterogeneous, non-parametric tests were used (Kruskal–Wallis and Mann–Whitney). The level of significance was set to p < 0.05. Correlations between denitrification/anammox and environmental factors were tested using the Pearson’s Correlation Coefficient (r). Statistical analyses were performed with Statistica 8 (StatSoft, Inc.) and R statistical package (R-Development Core Team 2011). All measurements are reported as average value with associated standard error.
Results
Physicochemical bottom water characteristics
Bottom water temperatures varied between 1.5 and 9.2 °C. Temperatures generally peaked in August and varied more at the inner than the outer stations (Table 1). Salinity ranged between 6.4 and 7.3 and was highest at station B1 and decreased towards the head of the estuary (Table 1).
At station B1, the lowest bottom water O2 concentration was recorded in May (140 μmol L−1), while at stations H2 and H4, the lowest concentrations (223 and 187 μmol L−1, respectively) occurred in August (Table 1). Hypoxia (O2 concentration <63 μmol L−1, as defined by Rabalais et al. (2010)) never appeared at these three stations during the sampling periods, whereas at station H6 hypoxic bottom waters occurred in October (55 μmol L−1, corresponding to 15 % saturation). Bottom water nutrient concentrations were slightly higher at station H6 than at the other stations (Table 1). Concentrations of NO2 − in the bottom water of Himmerfjärden were very low (always <0.8 μmol L−1) while concentrations of NO3 − ranged between 0.3 and 7.2 μmol L−1. NH4 + ranged between 0.4 and 16.1 μmol L−1, and PO4 3− peaked in October at H6 (7.4 μmol L−1), otherwise was always between 0.8 and 3.7 μmol L−1.
Sediment characteristics, isotopic composition and porewater profiles
Stations B1, H2 and H4 had bioturbated and oxidized sediment down to 5–7 cm depth below which the color was grey-black, and the sediment smelled of hydrogen sulfide. Sediments at B1 and H2 were colonized year-round by the polychaete Marenzelleria spp., whereas sediment at H4 was also inhabited by the bivalve Macoma balthica. Sediment porosities ranged between 0.85 and 0.91 at B1 and H2, while they were lower at H4 (0.77–0.85) (Table 1). At Station H6 the sediment was very aqueous and difficult to sample, with porosities ranging between 0.90 and 0.97 (Table 1). Here macrofauna was never observed and the surface sediment color changed over the year. In January the surface was characterized by a 1-cm thick brown layer; in October, however, the surface was covered by Beggiatoa mats. In June and August a very thin (1–3 mm) oxidized brown layer was present on the sediment surface.
The organic carbon content of the surface sediment was higher at stations B1 and H6 where it ranged between 4.3 and 6.1 % dry weight, whereas it was slightly lower (2.5–4.2 %) at stations H2 and H4 (Table 1). The molar C/N ratios in the surface sediment ranged from 7.9 to 9.8 (Table 1), and on an annual scale they were lower at H6 (8.4), intermediate at B1 and H2 (8.6 and 8.9, respectively) and higher at H4 (9.2). δ13C values were more negative at station H6 (range −26.4 to −24.9 ‰) than at the other three stations (range −24.8 to −23.6 ‰) (Table 1). δ15N values decreased toward the opening of the estuary and were between 6.8 and 8.4 ‰ at stations H6 and H4, between 5.1 and 5.6 ‰ at station H2, and between 3.9 and 4.5 ‰ at station B1 (Table 1).
Concentrations of nitrite (NO2 −) in the sediment porewater were very low and always <0.9 μmol L−1. Porewater concentrations of NO3 − at stations B1, H2, and H4 generally showed a peak in NO3 − in the topmost sediment layer (0–1 cm). This pattern was less evident at station B1 in May and absent in all porewater profiles from station H6, where NO3 − rapidly decreased from the surface of the sediment downward indicating minimal nitrification (data not shown). At stations B1, H2 and H4 porewater NH4 + concentrations increased considerably only below 2 cm depth, whereas at H6 (all samplings) and at B1 in May NH4 + immediately increased from the sediment surface downward (Fig. 2). Porewater profiles of DIP generally followed the trends found for NH4 + but the concentrations were always lower. In most cases the upper part of the profiles was linear and the diffusive fluxes of NH4 + and DIP could be calculated using the Fick’s first law of diffusion. N/P ratios higher than Redfield’s ratios occurred only at H4 in January (19.2), whereas minimum values were recorded at H6 in October, at the sediment surface (2.1) (Fig. 2).
Porewater profiles of ammonium (black squares), nitrate (grey rhombi) and phosphate (white circles). Nitrite concentration profiles are omitted (see text). First measurement from each profile represents a bottom water sample. N/P values indicate the average ratio between the concentrations of ammonium + nitrate + nitrite and phosphate (DIN/DIP) at three different depths along each profile. Note that profiles from May have a different Y-axis from the other profiles, and profiles from station H6 have a different X-axis for ammonium and phosphate
DIN and DIP fluxes, O2 microprofiles and uptake rates
NO2 − fluxes were negligible and always lower than 0.9 ± 0.4 μmol N m−2 h−1. NO3 − fluxes from the sediment were always positive at stations B1, H2 and H4 (range 3.2 to 21.5 μmol N m−2 h−1) whereas at station H6, NO3 − always diffused from the water column into the sediment (range −19.1 to −6.3 μmol N m−2 h−1), except for January when a low efflux was recorded (0.6 ± 0.4 μmol N m−2 h−1) (Table 3). The NH4 + fluxes were significantly higher (range 29.5–236.5 μmol N m−2 h−1) (p < 0.001) at station H6 than at the three outer stations. In October, when the bottom water was hypoxic at station H6, the efflux of NH4 + was three times higher compared to the other sampling periods (Table 3) whereas at stations B1, H2 and H4 the fluxes of NH4 + were not significantly different from each other (p > 0.05) and were always less than 9.7 ± 3.2 μmol N m−2 h−1. A comparison of diffusive NH4 + fluxes calculated from porewater depth gradients and rates measured by core incubations showed that the two methods only agreed well at station H6, except for October when measured fluxes were almost twice as high as the calculated ones (Table 3). At the other three stations there were more conspicuous differences (Table 3).
At station H6 DIP effluxes were high when they coincided with bottom hypoxia (October; 97.2 ± 11.9 μmol P m−2 h−1) and low when the bottom waters were oxic and bottom water temperatures were the highest (August; 12.0 ± 5.4 μmol P m−2 h−1). By contrast, the DIP flux was weakly negative, when the bottom waters were cold and oxic (January; −1.8 ± 0.2 μmol P m−2 h−1) (Table 3). At the other stations, the fluxes were positive throughout the year and were always lower than 3.3 ± 0.4 μmol P m−2 h−1, with little seasonal or spatial variation (p > 0.05). The fluxes of DIP calculated from porewater profiles and measured by core incubation were similar at station H6 (Table 3). Since macrofauna was absent at this muddy station throughout the year we assume that molecular diffusion was the dominant transport process. At the other stations, however, the measured whole-core fluxes were consistently higher than the calculated diffusive fluxes (Table 3). This observation is consistent with the higher abundance of bioturbating infauna at these stations.
Total O2 uptake (TOU) was generally higher at station B1 compared to stations H2, H4 and H6 (Table 3). TOU decreased from spring to winter at stations B1 (from −1,262 ± 165 to −396 ± 83 μmol O2 m−2 h−1) and H2 (from −927 ± 33 to −719 ± 77 μmol O2 m−2 h−1), whereas at H4 and H6 the maximum consumption was recorded in August (−757 ± 129 and −895 ± 58 μmol O2 m−2 h−1, respectively). At station H6 the seasonal pattern of TOUs differed from the other sites: the lowest TOU was measured in October (−233 ± 10 μmol O2 m−2 h−1) (Table 3), when O2 was low (55 μmol L−1). By contrast, O2 uptake was high (−648 ± 5 μmol O2 m−2 h−1) when bottom water oxygen was higher. The oxygen penetration depth (OPD) had a similar seasonal pattern at B1, H2, and H4 where it ranged between 0.25 and 0.38 cm in the period from June to October, with the minimum in August (Table 1). The OPD increased significantly to 0.61 and 0.90 cm in January at stations B1 and H4, respectively. At these stations, the diffusive oxygen uptake (DOU) was always lower than the TOU and ranged from −201 to −827 μmol O2 m−2 h−1 (Table 3). The differences between the in situ O2 value and the value at which the microprofiles were carried out were <20 % (Table 1), thus we consider the calculated DOU valid. In general, the ratio between TOU and DOU varied more between sampling periods than between the sampled sites (Table 3). TOU/DOU ratio was higher in June (1.9), and lower in August, October and January (1.2–1.3) (Table 3). The lowest OPDs were measured at station H6 ranging from 0.13 cm in October to 0.24 cm in January. At H6, due to significant differences (> 20 %) between the in situ O2 concentration and the value in the water overlying the sediment cores while profiling, it was only possible to calculate a reliable DOU (−549 μmol O2 m−2 h−1) and TOU/DOU ratio (1.1) for January (Table 3).
Anammox, denitrification, DNRA and nitrification
N2 production rates from intact sediment cores amended with 15NO3 − positively correlated (p < 0.05) with the 15NO3 − concentrations in May at H4 and in June at B1, H2 and H4, indicating that N2 was partly produced by anammox bacteria during these time periods. Anoxic slurries incubated with 15NH4 + revealed that anammox was always present at B1, H2 and H4. At station H6 p 29N2 was never detectable in anoxic slurries incubated with 15NH4 + (both with and without the addition of 14NO3 −), which indicates that anammox bacteria were not present or not active at this station. The values used in the calculations (V, r 14 and ra) are reported in Supplementary Table 1. Values for ra differed depending on the method used. In intact cores ra accounted for 0–19.1 %, while in anoxic slurries ra was lower (range from 0 to 10.6 %) (Fig. 3a). Slurry incubations may underestimate the anammox contribution to N2 production (Trimmer et al. 2006). Therefore in the text and the graphs of Fig. 3a we refer to N2 production rates preferentially from intact core incubations. When anammox was not detected with this method, N2 production and anammox contribution were estimated from the anoxic slurry experiments. When both methods failed in detecting anammox the standard IPT procedure was applied to calculate denitrification rates. Anammox rates were higher at H4 and H2 (ranging between 0.5 and 1.6 μmol N m−2 h−1) than at B1 (0.2–0.9 μmol N m−2 h−1) (Fig. 3a). Both anammox rates and ra negatively correlated with sedimentary organic C and N content (Table 4).
a Rates of N2 production divided in denitrification rates (den 14, black bars) and anammox rates (aao 14, grey bars). Error bars represent SE (n = 12). The percentage bars on the top represent the contribution of N2 production based on nitrate generated by nitrification (p 14 n, white shaded bars) and coming from the overlying water (p 14 w, grey shaded bars). b Rates of total DNRA. Error bars represent SE (n = 6). Note the different scale of the DNRA rates at station H6
Total N2 production in Himmerfjärden sediments was mainly sustained by denitrification (Fig. 3a). There was a pronounced seasonality in denitrification rates at stations B1, H2, and H4 (Fig. 3a), with higher rates measured in August (17.5 ± 0.8, 13.3 ± 0.7, 14.9 ± 0.5 μmol N m−2 h−1, respectively) than in May and January (< 6 μmol N m−2 h−1). At station H6 denitrification showed a different seasonal variation. Here den 14 was very low during the hypoxic period in October (1.7 ± 0.3 μmol N m−2 h−1), moderately low in June and August (4.0 ± 0.7, 8.3 ± 1.4, respectively), and highest in January (15.3 ± 0.8 μmol N m−2 h−1). At stations B1, H2 and H4 >80 % of the N2 production was sustained by nitrification (p 14 n) (Fig. 3a), while at station H6 p 14 n was only higher than p 14 w in January, when it was responsible for 72 % of total N2 loss. However, p 14 w dominated during the rest of the year at H6 (>59 %). At B1, H2 and H4 denitrification rates positively correlated with temperature and negatively with DOU, OPD and TOU (Table 4).
At station H6 rates of dissimilatory nitrate reduction to ammonium (DNRA) peaked at 29.9 ± 3.9 μmol N m−2 h−1 in October (Fig. 3b), which was seventeen times higher than the corresponding denitrification rates. In June and August DNRA rates were in the same order of magnitude as the N2 production rates (5.0 ± 1.2 and 3.20.7 μmol N m−2 h−1, respectively) and in January the DNRA rate was almost zero (0.3 ± 0.1 μmol N m−2 h−1). At the three less anthropogenically impacted stations the importance of DNRA was significantly lower (p < 0.001), the rates being always below 0.7 ± 0.1 μmol N m−2 h−1 and accounting for only 0–3.9 % of the total NO3 − reduction rates.
PNRs showed a seasonal trend similar to N2 removal via p 14 n (data not shown). At station B1, H2 and H4 PNRs did not vary significantly between seasons (p > 0.05) and they were always between 11.8 ± 0.2 and 26.7 ± 3.9 nmol N mL−1 h−1. Station H6 had similar PNRs rates in January (10.0 ± 0.6 nmol N mL−1 h−1), but negative conversion rates in August and October (−2.6 ± 1.6 and −3.8 ± 0.3 nmol N mL−1 h−1, respectively) indicating that NO3 − was not produced but was preferentially consumed during the experiment, therefore PNR represents net nitrification rates.
DE ranged between 39 and 45 % at stations B1, H2, and H4, whereas at H6 DE was only ~7 %. This implies that at H6 most of the regenerated NH4 + was recycled to the water column (Fig. 4).
Representation of principal annual N and P pathways and rates investigated in this study. All rates are reported as μmol m−2 h−1 and consist of annual average ± standard error. DE is denitrification efficiency and N/P indicates ratios of the annual average between DIN and DIP effluxes. Ammonification and nitrification were not measured in this study and were estimated from oxygen and nutrient fluxes (see “Materials and methods” section for details)
Discussion
Organic matter sources and benthic nitrogen and carbon mineralization
The negative stable carbon isotope signature (−26 ‰ vs. PDB) of sediment organic matter in the inner part of the estuary (station H6) suggests the dominance of terrestrially derived organic matter. Our measured values agree well with those of a previous study on the spatial distribution of stable N isotopes in Himmerfjärden sediments about 10 years ago (Savage et al. 2004). Since terrestrial runoff is minor in Himmerfjärden, this signal is most likely derived from the present and historical discharge of organic matter from the STP (Savage et al. 2004). Anthropogenic organic loading is also supported by the gradient of δ15N from the inner estuary at stations H4 and H6 to the outer stations, which can be attributed either to the influence of the STP discharge (Savage et al. 2004), to higher contribution of cyanobacterial biomass of sediment N at the outer stations H2 and B1, or to stronger NO3 − assimilation or denitrification (Brandes and Devol 1997) in the inner estuary. The latter process, however, can largely be excluded for most of the year close to the STP because heterotrophic denitrification was generally low in the estuary, and accounted for <5 % of the total carbon mineralization.
The denitrification rates reported in the present study are similar to those measured in the northern part of the Baltic Sea, the Gulf of Bothnia, but 50 % lower than those found in the southern Bothnian Sea (Stockenberg and Johnstone 1997). Our rates are at least twice as high as rates previously measured in sediment from the open Baltic Proper (Tuominen et al. 1998; Deutsch et al. 2010), generally comparable to those measured in the Gulf of Finland (Hietanen and Kuparinen 2008; Jäntti et al. 2011), but 1.5 to twofold lower than those in muddy sediments of the southern Baltic Sea (Deutsch et al. 2010). Compared to measurements in other coastal settings worldwide, our rates and those reported in previous studies from the Baltic are generally low (Seitzinger 1988; Steingruber et al. 2001). Denitrification rates did not correlate with organic carbon content, contrasting with the observations from a previous work on sedimentary denitrification in the Baltic (Deutsch et al. 2010). However, Deutsch et al. (2010) found a broader range of organic carbon concentrations at their stations than what we measured in Himmerfjärden. Moreover, bulk organic carbon does not discriminate between the easily degradable and more refractory pools and is therefore a poor proxy for carbon bioavailable for remineralization (Arnosti and Holmer 2003; Dittmar and Paeng 2009).
At stations B1, H2 and H4 differences in denitrification rates between sampling intervals are better explained by bottom water temperature, as suggested by the positive correlation between denitrification and temperature. In general this is expected since denitrification in marine sediments is a temperature dependent process (Rysgaard et al. 2004). On the other hand, our finding is in contrast with other studies from temperate estuaries and coastal systems, where lower denitrification rates were observed in summer during limiting NO3 − and O2 conditions (Rysgaard et al. 1995; Cabrita and Brotas 2000; Sundbäck et al. 2000). At B1, H2 and H4, however, O2 was never limiting and NO3 − for denitrifiers was mainly generated through nitrification. Nitrifiers from temperate sediments usually respond exponentially to temperature increase (Thamdrup and Fleischer 1998) and have a higher Q 10 than denitrifiers (Canfield et al. 2005). In our system denitrification was negatively correlated with OPDs, DOU and, to a lesser extent, with TOU suggesting that the large seasonal variations in denitrification/nitrification rates can be related to a tight coupling between bottom water temperature, oxygen uptake and penetration depth. In May and January the temperatures were the lowest, and the TOU was low as well implying a low flux of oxygen. The synergistic effect of these conditions might have limited nitrification rates and in turn denitrification. In addition, the deep OPD in January suggests that the amount of reactive organic material for heterotrophic denitrification was lower (Cai and Sayles 1996), which could also have lowered denitrification rates. Higher temperatures and high availability of NH4 + in the summer would have counteracted these effects by stimulating nitrifying bacteria (Canfield et al. 2005).
Oxygen consumption in Himmerfjärden sediments showed a strong seasonality and was affected by the variable oxygen concentrations in the bottom water, a feature typical of temperate marine coastal areas that continuously receive settling organic particles of terrestrial origin or from algal blooms and are characterized by significant seasonal temperature shifts (Bianchi 2007). The overall sediment oxygen uptake at all four sites was in the same order of magnitude as reported from the southern and the western Baltic (Balzer 1984; Jensen et al. 1995), the northern Baltic (Nedwell et al. 1983), and the transition between the Baltic and the North Sea (Jørgensen and Revsbech 1989).
Seasonal changes in temperature, the chemical environment of the porewaters, and the resultant effects on macrofaunal activity can explain a shift in the TOU/DOU ratio from 1.9 in June to nearly 1 for the rest of the year at stations B1, H2, and H4. It is likely that macrofauna was much more abundant and active in late spring, while it was negatively affected by reducing conditions during the summer and autumn. DOU could not be calculated at station H6 except from January when the ratio was ca. 1. Here hypoxia and highly reducing chemical conditions made the environment hostile to macrofauna and solute exchange across the interface was largely diffusive.
Although the oxygen consumption rates at the four stations were of the same order of magnitude, NH4 + effluxes were 10- to 400-times higher at station H6 than at the less impacted sites. Since C/N ratios and aerobic degradation processes in the surface sediments of all stations were comparable, NH4 + removal processes such as nitrification and anammox were more relevant in the surface sediment of stations B1, H2, and H4 than at station H6, and DNRA and deep anaerobic mineralization of organic matter and ammonification were higher at H6. This is also supported by the observation that calculated production rates of NH4 + (assuming a respiratory quotient of 1.2 and C/N ratios between 7.9 and 9.3, see Table 1) at station B1, H2 and H4 were much higher than the measured fluxes to the bottom water. An agreement between calculated NH4 + production rates and measured fluxes was only observed at station H6, which suggests that nitrification and anammox were inhibited at this site.
N transformation pathways in Himmerfjärden
An important difference between stations B1, H2, H4 and station H6 is the direction of the NO3 − flux, which was of the same order of magnitude, but out of the sediment at stations B1, H2, H4 and into the sediment at station H6. This implies that at B1, H2 and H4 a significant fraction (~25 %) of the NH4 + produced via ammonification was coupled to nitrification (Henriksen et al. 1981). However, a large fraction (69–78 %) of the NH4 + was neither nitrified nor lost to the water column as dissolved NH4 + (Fig. 4). This fraction could have been retained by adsorption, assimilated in the sediment by bacteria, or could have been lost from the sediment in the form of DON (Dalsgaard 2003). NH4 + adsorption was limited at station H6 because the sediment porosity was close to one, and the sites of NH4 + exchange were likely saturated (Mackin and Aller 1984). The mismatch between NH4 + production and NH4 + loss may also suggest that our TOU rates overestimated the total mineralization rates, and that a significant fraction of the consumed O2 was used for chemical and biological oxidation of reduced inorganic compounds that were produced by anaerobic metabolism and/or accumulated during hypoxic periods (Christensen et al. 1987).
At B1, H2 and H4 about half of the produced NO3 − was released to the water column while the other half was consumed via coupled nitrification–denitrification (Fig. 4). At H6 only a very small amount of the produced NH4 + was nitrified (<4 %) and lost via p 14 n. Surprisingly, on an annual scale, the loss of fixed N as N2 was comparable at the four sampling sites (~90 mmol N m−2 year−1) but at B1, H2 and H4 this amount was almost entirely derived from p 14 n due to low concentrations of NO3 − in the water column and relatively stable oxic bottom water conditions. At station H6 p 14 w contributed more than p 14 n to total denitrification likely due to higher concentrations of NO3 − supplied by the STP and more reducing sediments compared to the other sites (Rysgaard et al. 1994). Here, fixed N was lost via denitrification mainly during winter while in summer/autumn N was efficiently recycled in the system (Kemp et al. 2005).
The data from Station H6 deserve special attention. DNRA was not a relevant process at stations B1, H2 and H4, but at the impacted station H6 in October DNRA rates were significantly higher than rates of denitrification, a typical characteristic of sulfidic, organic-rich sediments (Christensen et al. 2000; Nizzoli et al. 2006). Here, bottom water oxygen concentrations played a major role in the regulation of NO3 − reduction pathways: when oxygen was abundant, the black, sulfidic sediment surface was replaced by a thin layer of light-brown oxidized sediment in which nitrification could take place. The reoxygenation of the sediment surface stimulated nitrification and allowed denitrifying bacteria to reduce the regenerated NO3 − pool to N2. As a consequence, at this site denitrification rates were highest in the winter when the temperatures were the lowest, and mostly supported by nitrification–denitrification. Nitrifying microorganisms have been shown to grow slower than denitrifiers both in sediments and in bioreactors (Dollhopf et al. 2005; Duan et al. 2009). Our findings suggest that the nitrifying microorganisms in this sediment either grew as rapidly as the denitrifying bacteria despite the low temperatures or that a large dormant nitrifying population was reactivated as oxygen concentrations increased.
When the bottom waters at station H6 were hypoxic and in situ NO3 − concentrations were low (i.e., October), DNRA was the dominant NO3 − reduction pathway. The disappearance of the superficial oxic zone coincided with the suppression of nitrification and, consequently, denitrification. It is likely that the high concentrations of dissolved sulfide close to the sediment surface in the inner part of Himmerfjärden (Thang et al. 2013) suppressed nitrification. Observations of the surface sediment showed that it was black and populated by Beggiatoa spp, also suggesting high sulfide concentrations. These conditions favor chemolithoautotrophic DNRA over heterotrophic denitrification and DNRA; Beggiatoa and other sulfide oxidizers can couple the reduction of NO3 − to NH4 + with sulfide oxidation (Brunet and Garcia-Gil 1996; Jørgensen and Nelson 2004), whereas sulfide can inhibit denitrification (Sørensen et al. 1980).
The results from the potential nitrification experiments further support these interpretations. Despite the presence of oxygen, NH4 + was not oxidized in sediment from station H6 over the duration of the experiment. Instead, the slurry experiments in August and October indicated partial denitrification in the presence of oxygen: the average rate of NO3 − consumption was 3.2 nmol N mL−1 h−1 while nitrite accumulation was 3.0 nmol N mL−1 h−1 and NH4 + accumulated at rates of 0.5 nmol N mL−1 h−1. Slurry experiments from the other three stations always showed high potential NO3 − production rates, and no accumulation of nitrite at the end of the experiments. DNRA rates were negligible at these stations and always lower than 1 μmol N m−2 h−1 indicating that NH4 + was efficiently converted to NO3 −.
High DNRA activity in sediment cores incubated with 15NO3 − can interfere with the calculation of p 14 because it supplies the system with 15NH4 + that can be used by anammox bacteria, resulting in anammox underestimation and p 14 overestimation (Hietanen 2007). This effect was likely not significant in our case because anammox was not present where DNRA rates were high (station H6). Where anammox activity was detected (stations B1, H2 and H4) DNRA rates were low and they unlikely affected the ratio between 14NO3 − and 15NO3 − in the NO3 − reduction zone. With anoxic slurry incubations the same problem can arise when DNRA takes part in NO3 − reduction, because the 15NH4 + produced by DNRA in anoxic slurries incubated with 15NO3 − would lead to p 30N2 by anammox, and the assumption that p 30N2 is produced only by denitrification would fail (Thamdrup and Dalsgaard 2002; Song et al. 2013). However, H6 sediment did not show any production of 29N2 in anoxic slurries incubated with 15NH4 + + 14NO3 −. At the other stations, potential anammox rates calculated from slurries incubated with either 15NH4 + + 14NO3 − or 15NO3 − were the same, meaning that DNRA did not supply anammox with any extra 15NH4 + in the latter case. This could be due to the fact that even if DNRA was partially present at these stations, its effect in the anoxic slurries was either 1) masked by the high background ammonium concentrations (Song et al. 2013); 2) inhibited by the strong additions of NO3 − (150 μM) to the slurries, as it was shown that nitrate ammonifiers are active in conditions where NO3 − is limiting (Smith 1982; Bonin 1996; Kelso et al. 1997).
Rates of anammox and denitrification from slurry incubations have to be considered potential because the nutrient concentrations were exaggerated (150 μmol N L−1), the amount of sediment used was small (2 mL in each vial), and the sediment structure was likely altered during sediment slicing and homogenization (Trimmer et al. 2006). Therefore in this study potential rates from slurry incubations were not used apart from calculating the relative importance of anammox to N2 production, when intact core incubations failed in detecting anammox. Anammox rates and anammox contribution to N2 production were negatively correlated with organic carbon content, which indicate that in sediments with lower organic carbon contents anammox bacteria had a greater competitive advantage, possibly because of lesser substrate competition with denitrifying bacteria (Dalsgaard et al. 2005). Whether anammox contributes significantly to N2 production in the Baltic sediments has been controversial. Deutsch et al. (2010) did not find anammox in the south and central Baltic Sea using sediment cores amended with different concentrations of 15NO3 −. However, as our data show, anammox cannot be totally excluded, due to the fact that this method seems not to be effective at low anammox activities (Supplementary Table 1). Anammox was not measured previously in Himmerfjärden, and the rates measured in this study were low (generally <1.5 μmol m−2 h−1) but comparable to those from other Baltic Sea coastal areas in the Gulf of Finland (Hietanen 2007; Hietanen and Kuparinen 2008; Jäntti et al. 2011).
Previously reported denitrification rates in Himmerfjärden were on average 33 μmol m−2 h−1 (Elmgren and Larsson 1997), which is a factor three higher than the rates found in this study. Although it is possible that this difference is due to the fact that in this earlier study sediment was taken from shallower depth where temperature and deposition of fresh organic matter may have been higher, we consider this explanation less likely. More importantly, when these earlier experiments were carried out, the DIN discharge from the STP was three times higher than today (763 t N year−1 in 1988–1989 compared to 230 t N year−1 in 2011) and bottom water hypoxia was more widespread than today. This would have led to significantly higher bottom water NO3 − levels and p 14 w rates. It is conspicuous that the threefold drop in denitrification agrees so well with the decrease in DIN discharge from the STP. The drop in denitrification rates could be even more significant when considering that the acetylene inhibition method used in the Elmgren and Larsson (1997) study tends to underestimate rates of denitrification because of potential co-inhibition of nitrifying bacteria (Steingruber et al. 2001). Furthermore, in the 1980s and early 1990s the sediment hosted different communities of bioturbating fauna dominated by the amphipods Monoporeia affinis and Pontoporeia femorata (Elmgren and Larsson 1997), whereas today the widespread polychaete Marenzelleria spp. dominates benthic macrofauna (Elmgren and Larsson 1997; Karlson et al. 2011). Karlson et al. (2005) showed that the bioturbation activity of M. affinis stimulated N2 removal compared to non-bioturbated sediment, whereas recent experiments with conditioned sediment with and without Marenzelleria spp. demonstrated that denitrification rates were even lower in the presence of the polychaete (Bonaglia et al. 2013).
Stoichiometry of N and P regeneration
On an annual scale, molar N/P ratios of benthic fluxes were 9.7 ± 2.5 at station B1, 7.6 ± 1.6 at station H2, 8.0 ± 1.8 at station H4, and 4.2 ± 0.6 at station H6. The N/P ratios of additional external N sources for Himmerfjärden are insufficient to reach a Redfield N/P ratio of 16 (Savage et al. 2002). Since the DIN effluxes reported here are in the range of values from previous studies of Baltic sediments (Tuominen et al. 1999), we attribute the low N/P ratios to the high PO4 3− effluxes. To our knowledge, the DIP efflux measured at station H6 in October (97.2 μmol P m−2 h−1) is the highest reported for any Baltic Sea station to date. There are no published sediment DIP fluxes from Himmerfjärden to compare to our data, but the DIP fluxes and N/P ratios measured from hypoxic/anoxic Baltic Sea sediments were in the range of the lower rates at station H6 (Viktorsson et al. 2013). Interestingly, during our sampling campaign this station was never anoxic but only seasonally hypoxic suggesting that DIP fluxes could be even higher under fully anoxic conditions (Viktorsson et al. 2012). Strongly reducing conditions and dissolved sulfide appearing close to the sediment surface in the inner part of the estuary (Thang et al. 2013) would have quickly consumed the oxygen present in the near bottom water. In turn, this would have limited the formation of iron oxyhydroxides and, therefore, limited the scavenging of dissolved P. The high fluxes (and the low N/P ratios) we measured in the inner Himmerfjärden can be thus related to high organic matter supply, high mineralization rates, and low retention of P by metal oxides as was suggested for other coastal areas in the Baltic Sea (Lukkari et al. 2009).
Conclusions and ecosystem implications
We assessed the degree of sedimentary N loss and the stoichiometry of the regenerated N and P along a eutrophication gradient showing that hypoxia and high nutrient loading—recurring conditions in the inner part of the estuary—favor N and P recycling over N loss and P retention (Christensen et al. 2000; Kemp et al. 2005). This has a positive feedback for eutrophication and the potential recurrence for summer cyanobacteria blooms. However, two out of three stations situated in Himmerfjärden have very similar rates and N cycling processes relative to the “control” station, situated outside the estuary and representing relatively pristine coastal sediments. These sites did not have significantly different sediment types and organic carbon contents. An interesting implication is that the anthropogenic discharge 9 km away from the STP had little effect on the microbial nitrogen transformation pathways, albeit not necessarily the rates. Apparently, at these sites, seasonal variations in temperature, together with oxygen uptake and penetration depth, exerted the strongest control on nitrification, which in turn regulated denitrification rates. This confirms that in temperate coastal areas with low NO3 − concentrations in the water column, seasonal variations in bottom water temperature might be an important environmental factor in controlling N cycling processes (Hietanen and Kuparinen 2008).
We estimated the sedimentary N loss and the internal N and P loadings by multiplying the annual rates of N2 production and the nutrient fluxes by the surface areas of the sub-basins within Himmerfjärden reported by Engqvist and Omstedt (1992). About 383 t N year−1 are recycled between the sediment and the water column in the system mainly through benthic NH4 + efflux. This value is significantly lower than the external sources of N from the catchment area (runoff, input from the upper lake Mälaren, and STP) that were estimated to be approximately 850 t year−1 (Savage et al. 2002). By contrast, the calculated internal loading of P (132 t year−1) was almost three times higher than the external loading of 45 t P year−1 reported by Savage et al. (2002). The coastal sediments are therefore currently the major source of P for the Himmerfjärden waters, where they enhance primary production, and contribute to the blooms of N-fixing cyanobacteria.
The calculations also indicate that 221 t of N year−1 are permanently removed by denitrification (and, to a minor extent, by anammox) from the estuary, which is comparable to approximately 96 % of the DIN discharged annually by the Himmerfjärden STP. Considering the significantly higher denitrification rates during the late 1980’s (Elmgren and Larsson 1997) when the anthropogenic DIN discharge to the estuary was threefold higher, these new data suggest that the Himmerfjärden estuarine system operates as an efficient catalyst, removing N as N2 from anthropogenic and land-derived sources at loadings up to three times of the present N loading. We conclude that in the current situation denitrification in estuarine sediments is an efficient N sink for external N inputs, and acts as a buffer between land and the open ocean (Seitzinger 1988; Gruber and Galloway 2008). However, under expanding or permanent hypoxia, estuaries could undergo shifts to efficient N recycling, which would reduce the natural N loss and exacerbate eutrophication.
References
An S, Gardner WS (2002) Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar Ecol Prog Ser 237:41–50
Arnosti C, Holmer M (2003) Carbon cycling in a continental margin sediment: contrasts between organic matter characteristics and remineralization rates and pathways. Estuar Coast Shelf Sci 58(1):197–208
Balzer W (1984) Organic matter degradation and biogenic element cycling in a nearshore sediment (Kiel Bight). Limnol Oceanogr 29(6):1231–1246
Berg P, Risgaard-Petersen N, Rysgaard S (1998) Interpretation of measured concentration profiles in sediment pore water. Limnol Oceanogr 43(7):1500–1510
Bianchi TS (2007) Biogeochemistry of estuaries. Oxford University Press, Oxford
Bonaglia S, Bartoli M, Gunnarsson JS, Rahm L, Raymond C, Svensson O, Shakeri Yekta S, Brüchert V (2013) Effect of reoxygenation and Marenzelleria spp. bioturbation on Baltic Sea sediment metabolism. Mar Ecol Prog Ser 482:43–55
Bonin P (1996) Anaerobic nitrate reduction to ammonium in two strains isolated from coastal marine sediment: a dissimilatory pathway. FEMS Microbiol Ecol 19(1):27–38
Brandes JA, Devol AH (1997) Isotopic fractionation of oxygen and nitrogen in coastal marine sediments. Geochim Cosmochim Acta 61(9):1793–1801
Brunet RC, Garcia-Gil LJ (1996) Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol Ecol 21(2):131–138
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5(2):89–96
Cabrita MT, Brotas V (2000) Seasonal variation in denitrification and dissolved nitrogen fluxes in intertidal sediments of the Tagus estuary, Portugal. Mar Ecol Prog Ser 202:51–65
Cai W-J, Sayles FL (1996) Oxygen penetration depths and fluxes in marine sediments. Mar Chem 52(2):123–131
Canfield DE, Kristensen E, Thamdrup B (2005) The nitrogen cycle. In: Canfield DE, Kristensen E, Thamdrup B (eds) Aquatic Geomicrobiology. Academic Press, San Deigo, pp 205–267
Christensen JP, Smethie WM Jr, Devol AH (1987) Benthic nutrient regeneration and denitrification on the Washington continental shelf. Deep-Sea Res I 34(5–6):1027–1047
Christensen PB, Rysgaard S, Sloth NP, Dalsgaard T, Schwærter S (2000) Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquat Microb Ecol 21(1):73–84
Conley DJ, Bjorck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson BG, Hietanen S, Kortekaas M, Kuosa H, Meier HEM, Muller-Karulis B, Nordberg K, Norkko A, Nurnberg G, Pitkanen H, Rabalais NN, Rosenberg R, Savchuk OP, Slomp CP, Voss M, Wulff F, Zillen L (2009) Hypoxia-related processes in the Baltic Sea. Environ Sci Technol 43(10):3412–3420
Conley DJ, Carstensen J, Aigars J, Axe P, Bonsdorff E, Eremina T, Haahti BM, Humborg C, Jonsson P, Kotta J, Lannegren C, Larsson U, Maximov A, Medina MR, Lysiak-Pastuszak E, Remeikaite-Nikiene N, Walve J, Wilhelms S, Zillen L (2011) Hypoxia is increasing in the coastal zone of the Baltic Sea. Environ Sci Technol 45(16):6777–6783
Dalsgaard T (2003) Benthic primary production and nutrient cycling in sediments with benthic microalgae and transient accumulation of macroalgae. Limnol Oceanogr 48(6):2138–2150
Dalsgaard T, Nielsen LP, Brotas V, Viaroli P, Underwood G, Nedwell DB, Sundbäck K, Rysgaard S, Miles A, Bartoli M, Dong L, Thornton DCO, Ottosen LDM, Castaldelli G, Risgaard-Petersen N (2000) In: Dalsgaard T (ed) Protocol handbook for NICE: Nitrogen cycling in estuaries: a project under the EU research programme: Marine Science and Technology (MAST III). National Environmental Research Institute, Silkeborg
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156(4):457–464
Dalsgaard T, De Brabandere L, Hall POJ (2013) Denitrification in the water column of the central Baltic Sea. Geochim Cosmochim Acta 106:247–260
Deutsch B, Forster S, Wilhelm M, Dippner JW, Voss M (2010) Denitrification in sediments as a major nitrogen sink in the Baltic Sea: an extrapolation using sediment characteristics. Biogeosciences 7(10):3259–3271
Dittmar T, Paeng J (2009) A heat-induced molecular signature in marine dissolved organic matter. Nature Geosci 2(3):175–179
Dollhopf SL, Hyun JH, Smith AC, Adams HJ, O’Brien S, Kostka JE (2005) Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Appl Environ Microbiol 71(1):240–246
Dong LF, Sobey MN, Smith CJ, Rusmana I, Phillips W, Stott A, Osborn AM, Nedwell DB (2011) Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol Oceanogr 56(1):279–291
Duan L, Moreno-Andrade I, Huang C-l, Xia S, Hermanowicz SW (2009) Effects of short solids retention time on microbial community in a membrane bioreactor. Bioresour Technol 100(14):3489–3496
Duarte CM, Conley DJ, Carstensen J, Sánchez-Camacho M (2009) Return to Neverland: shifting baselines affect eutrophication restoration targets. Estuar Coast 32(1):29–36
Elmgren R (2001) Understanding human impact on the Baltic ecosystem: changing views in recent decades. Ambio 30(4–5):222–231
Elmgren R, Larsson U (1997) Himmerfjärden: förändringar i ett näringsbelastat kustekosystem i Östersjön. In: Naturvårdsverket Förlag S, Sweden (Summary in English) (ed) p 197
Engqvist A, Omstedt A (1992) Water exchange and density structure in a multi-basin estuary. Cont Shelf Res 12(9):1003–1026
Eyre BD, Ferguson AJP (2009) Denitrification efficiency for defining critical loads of carbon in shallow coastal ecosystems. Hydrobiologia 629(1):137–146
Gardner WS, McCarthy MJ, An S, Sobolev D, Sell KS, Brock D (2006) Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol Oceanogr 51(1):558–568
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451(7176):293–296
Henriksen K, Hansen JI, Blackburn TH (1981) Rates of nitrification, distribution of nitrifying bacteria, and nitrate fluxes in different types of sediment from Danish waters. Mar Biol 61(4):299–304
Hietanen S (2007) Anaerobic ammonium oxidation (anammox) in sediments of the Gulf of Finland. Aquat Microb Ecol 48(2):197–205
Hietanen S, Kuparinen J (2008) Seasonal and short-term variation in denitrification and anammox at a coastal station on the Gulf of Finland, Baltic Sea. Hydrobiologia 596:67–77
Hietanen S, Jäntti H, Buizert C, Jürgens K, Labrenz M, Voss M, Kuparinen J (2012) Hypoxia and nitrogen processing in the Baltic Sea water column. Limnol Oceanogr 57(1):325–337
Holtappels M, Lavik G, Jensen MM, Kuypers MMM (2011) 15N-labeling experiments to dissect the contributions of heterotrophic denitrification and anammox to nitrogen removal in the OMZ waters of the ocean. In: Martin GK (ed) Methods in Enzymology, vol 486. Academic Press, San Deigo, pp 223–251
Hulth S, Aller RC, Canfield DE, Dalsgaard T, Engström P, Gilbert F, Sundbäck K, Thamdrup B (2005) Nitrogen removal in marine environments: recent findings and future research challenges. Mar Chem 94(1–4):125–145
Iversen N, Jørgensen BB (1993) Diffusion coefficients of sulfate and methane in marine sediments: influence of porosity. Geochim Cosmochim Acta 57(3):571–578
Jäntti H, Hietanen S (2012) The effects of hypoxia on sediment nitrogen cycling in the Baltic Sea. Ambio 41(2):161–169
Jäntti H, Stange F, Leskinen E, Hietanen S (2011) Seasonal variation in nitrification and nitrate-reduction pathways in coastal sediments in the Gulf of Finland, Baltic Sea. Aquat Microb Ecol 63(2):171–181
Jensen K, Sloth NP, Risgaard-Petersen N, Rysgaard S, Revsbech NP (1994) Estimation of nitrification and denitrification from microprofiles of oxygen and nitrate in model sediment systems. Appl Environ Microbiol 60(6):2094–2100
Jensen HS, Mortensen PB, Andersen FO, Rasmussen E, Jensen A (1995) Phosphorus cycling in a coastal marine sediment, Aarhus Bay, Denmark. Limnol Oceanogr 40(5):908–917
Jørgensen BB, Nelson DC (2004) Sulfide oxidation in marine sediments: Geochemistry meets microbiology. In: Amend JP, Edwards KJ, Lyons TW (eds) Sulfur Biogeochemistry: Past and Present. Geological Society of America Special Papers. pp 63–81
Jørgensen BB, Revsbech NP (1989) Oxygen uptake, bacterial distribution, and carbon-nitrogen-sulfur cycling in sediments from the Baltic Sea: North Sea transition. Ophelia 31(1):29–49
Karlson K, Hulth S, Ringdahl K, Rosenberg R (2005) Experimental recolonisation of Baltic Sea reduced sediments: survival of benthic macrofauna and effects on nutrient cycling. Mar Ecol Prog Ser 294:35–49
Karlson AML, Näslund J, Rydén SB, Elmgren R (2011) Polychaete invader enhances resource utilization in a species-poor system. Oecologia 166(4):1055–1065
Kelso B, Smith RV, Laughlin RJ, Lennox SD (1997) Dissimilatory nitrate reduction in anaerobic sediments leading to river nitrite accumulation. Appl Environ Microbiol 63(12):4679–4685
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303:1–29
Löffler A, Schneider B, Schmidt M, Nausch G (2011) Estimation of denitrification in Baltic Sea deep water from gas tension measurements. Mar Chem 125(1–4):91–100
Lukkari K, Leivuori M, Kotilainen A (2009) The chemical character and behaviour of phosphorus in poorly oxygenated sediments from open sea to organic-rich inner bay in the Baltic Sea. Biogeochemistry 96(1–3):25–48
Mackin JE, Aller RC (1984) Ammonium adsorption in marine sediments. Limnol Oceanogr 29(2):250–257
Meier HEM, Müller-Karulis B, Andersson H, Dieterich C, Eilola K, Gustafsson B, Höglund A, Hordoir R, Kuznetsov I, Neumann T, Ranjbar Z, Savchuk O, Schimanke S (2012) Impact of climate change on ecological quality indicators and biogeochemical fluxes in the Baltic Sea: a multi-model ensemble study. Ambio 41(6):558–573
Nedwell DB, Hall S-E, Andersson A, Hagström ÅF, Lindström EB (1983) Seasonal changes in the distribution and exchange of inorganic nitrogen between sediment and water in the Northern Baltic (Gulf of Bothnia). Estuar Coast Shelf Sci 17(2):169–179
Neubacher EC, Parker RE, Trimmer M (2011) Short-term hypoxia alters the balance of the nitrogen cycle in coastal sediments. Limnol Oceanogr 56(2):651–665
Neubacher E, Parker R, Trimmer M (2013) The potential effect of sustained hypoxia on nitrogen cycling in sediment from the southern North Sea: a mesocosm experiment. Biogeochemistry 113(1–3):69–84
Nielsen LP (1992) Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiol Ecol 86(4):357–362
Nizzoli D, Welsh DT, Fano EA, Viaroli P (2006) Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Mar Ecol Prog Ser 315:151–165
Rabalais NN, Diaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J (2010) Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7(2):585–619
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org
Risgaard-Petersen N, Rysgaard S (1995) Nitrate reduction in sediments and waterlogged soil measured by 15N techniques. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 287–295
Risgaard-Petersen N, Rysgaard S, Revsbech NP (1995) Combined microdiffusion-hypobromite oxidation method for determining nitrogen-15 isotope in ammonium. Soil Sci Soc Am J 59(4):1077–1080
Risgaard-Petersen N, Nielsen LP, Rysgaard S, Dalsgaard T, Meyer RL (2003) Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnol Oceanogr Methods 1:63–73
Rönner U (1985) Nitrogen transformations in the Baltic Proper: Denitrification counteracts eutrophication. Ambio 14(3):134–138
Rysgaard S, Risgaard-Petersen N, Sloth NP, Jensen K, Nielsen LP (1994) Oxygen regulation of nitrification and denitrification in sediments. Limnol Oceanogr 39(7):1643–1652
Rysgaard S, Christensen PB, Nielsen LP (1995) Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Mar Ecol Prog Ser 126(1–3):111–121
Rysgaard S, Glud RN, Risgaard-Petersen N, Dalsgaard T (2004) Denitrification and anammox activity in arctic marine sediments. Limnol Oceanogr 49(5):1493–1502
Savage C, Elmgren R, Larsson U (2002) Effects of sewage-derived nutrients on an estuarine macrobenthic community. Mar Ecol Prog Ser 243:67–82
Savage C, Leavitt PR, Elmgren R (2004) Distribution and retention of effluent nitrogen in surface sediments of a coastal bay. Limnol Oceanogr 49(5):1503–1511
Schulz H (2006) Quantification of early diagenesis: Dissolved constituents in pore water and signals in the solid phase. In: Schulz H, Zabel M (eds) Marine geochemistry. Springer, Berlin, pp 73–124
Seitzinger SP (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33(4):702–724
Smith MS (1982) Dissimilatory reduction of NO2 − to NH4 + and N2O by a soil Citrobacter sp. Appl Environ Microbiol 43(4):854–860
Solorzano L (1969) Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14(5):799–801
Song GD, Liu SM, Marchant H, Kuypers MMM, Lavik G (2013) Anaerobic ammonium oxidation, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosci Discuss 10(3):4671–4710
Sørensen J, Tiedje JM, Firestone RB (1980) Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol 39(1):105–108
Steingruber SM, Friedrich J, Gachter R, Wehrli B (2001) Measurement of denitrification in sediments with the 15N isotope pairing technique. Appl Environ Microbiol 67(9):3771–3778
Stockenberg A, Johnstone RW (1997) Benthic denitrification in the Gulf of Bothnia. Estuar Coast Shelf Sci 45(6):835–843
Sundbäck K, Miles A, Göransson E (2000) Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: an annual study. Mar Ecol Prog Ser 200:59–76
Sundby B, Gobeil C, Silverberg N, Mucci A (1992) The phosphorus cycle in coastal marine sediments. Limnol Oceanogr 37(6):1129–1145
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68(3):1312–1318
Thamdrup B, Fleischer S (1998) Temperature dependence of oxygen respiration, nitrogen mineralization, and nitrification in Arctic sediments. Aquat Microb Ecol 15(2):191–199
Thang N, Brüchert V, Formolo M, Wegener G, Ginters L, Jørgensen BB, Ferdelman TG (2013) The impact of sediment and carbon fluxes on the biogeochemistry of methane and sulfur in littoral Baltic Sea sediments (Himmerfjärden, Sweden). Estuar Coast 36(1):98–115
Trimmer M, Risgaard-Petersen N, Nicholls JC, Engstrom P (2006) Direct measurement of anaerobic ammonium oxidation (anammox) and denitrification in intact sediment cores. Mar Ecol Prog Ser 326:37–47
Tuominen L, Heinanen A, Kuparinen J, Nielsen LP (1998) Spatial and temporal variability of denitrification in the sediments of the northern Baltic Proper. Mar Ecol Prog Ser 172:13–24
Tuominen L, Makela K, Lehtonen KK, Haahti H, Hietanen S, Kuparinen J (1999) Nutrient fluxes, porewater profiles and denitrification in sediment influenced by algal sedimentation and bioturbation by Monoporeia affinis. Estuar Coast Shelf Sci 49(1):83–97
Vahtera E, Conley DJ, Gustafsson BG, Kuosa H, Pitkanen H, Savchuk OP, Tamminen T, Viitasalo M, Voss M, Wasmund N, Wulff F (2007) Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 36(2–3):186–194
Viktorsson L, Almroth-Rosell E, Tengberg A, Vankevich R, Neelov I, Isaev A, Kravtsov V, Hall PJ (2012) Benthic phosphorus dynamics in the Gulf of Finland, Baltic Sea. Aquat Geochem 18(6):543–564
Viktorsson L, Ekeroth N, Nilsson M, Kononets M, Hall POJ (2013) Phosphorus recycling in sediments of the central Baltic Sea. Biogeosciences 10(6):3901–3916
Zillén L, Conley DJ, Andrén T, Andrén E, Björck S (2008) Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth-Sci Rev 91(1–4):77–92
Acknowledgments
We thank the staff of the Askö laboratory, Livija Ginters, Joanna Sawicka and Linnea Isaksson for their assistance during sampling. We acknowledge Heike Siegmund from Stable Isotope Laboratory (Stockholm University) for analysis of stable isotopes of organic C and N, and the staff of the chemical laboratory at Department of Systems Ecology (Stockholm University) for nutrient analysis. We acknowledge three anonymous reviewers and the editor for their valuable comments. Financial support for this study was provided by the Formas Strategic Research projects Managing Baltic nutrients (V.B.) and Baltic Ecosystem Adaptive Management—BEAM (V.B.), and by a grant from Stockholm University Marine research Centre (S.B.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Mark Brush
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bonaglia, S., Deutsch, B., Bartoli, M. et al. Seasonal oxygen, nitrogen and phosphorus benthic cycling along an impacted Baltic Sea estuary: regulation and spatial patterns. Biogeochemistry 119, 139–160 (2014). https://doi.org/10.1007/s10533-014-9953-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-014-9953-6