Abstract
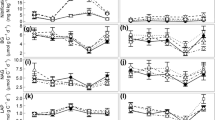
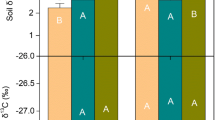
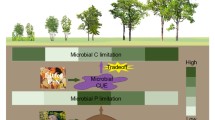
Soil extracellular enzymes mediate organic matter turnover and nutrient cycling yet remain little studied in one of Earth’s most rapidly changing, productive biomes: tropical forests. Using a long-term leaf litter and throughfall manipulation, we explored relationships between organic matter (OM) inputs, soil chemical properties and enzyme activities in a lowland tropical forest. We assayed six hydrolytic soil enzymes responsible for liberating carbon (C), nitrogen (N) and phosphorus (P), calculated enzyme activities and ratios in control plots versus treatments, and related these to soil biogeochemical variables. While leaf litter addition and removal tended to increase and decrease enzyme activities per gram soil, respectively, shifts in enzyme allocation patterns implied changes in relative nutrient constraints with altered OM inputs. Enzyme activity ratios in control plots suggested strong belowground P constraints; this was exacerbated when litter inputs were curtailed. Conversely, with double litter inputs, increased enzymatic investment in N acquisition indicated elevated N demand. Across all treatments, total soil C correlated more strongly with enzyme activities than soluble C fluxes, and enzyme ratios were sensitive to resource stoichiometry (soil C:N) and N availability (net N mineralization). Despite high annual precipitation in this site (MAP ~5 m), soil moisture positively correlated with five of six enzymes. Our results suggest resource availability regulates tropical soil enzyme activities, soil moisture plays an additional role even in very wet forests, and relative investment in C, N and P degrading enzymes in tropical soils will often be distinct from higher latitude ecosystems yet is sensitive to OM inputs.


Similar content being viewed by others
References
Aber JD, Melillo JM (1980) Litter decomposition—measuring relative contributions of organic-matter and nitrogen to forest soils. Can J Bot (Revue Canadienne De Botanique) 58(4):416–421
Allison SD (2006) Soil minerals and humic acids alter enzyme stability: implications for ecosystem processes. Biogeochemistry 81(3):361–373
Allison SD, Vitousek PM (2004) Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36(3):285–296
Allison S, Wallenstein M, Bradford M (2010) Soil-carbon response to warming dependent on microbial physiology. Nat Geosci 3:336–340
Austin A, Vitousek P (1998) Nutrient dynamics on a precipitation gradient in Hawai’i. Oecologia 113:519–529
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomass C. Soil Biol Biochem 29(7):1023–1032
Berg B (2000) Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133(1–2):13–22
Bern CR, Townsend AR, Farmer GL (2005) Unexpected dominance of parent-material strontium in a tropical forest on highly weathered soils. Ecology 86(3):626–632
Berrange JP, Thorpe RS (1988) The geology, geochemistry and emplacement of the cretaceous tertiary ophiolitic Nicoya Complex of the Osa Peninsula, southern Costa-Rica. Tectonophysics 147(3–4):193–220
Bonan GB (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320(5882):1444–1449
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil-nitrogen—a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17(6):837–842
Brzostek ER, Finzi AC (2011) Substrate supply, fine roots, and temperature control proteolytic enzyme activity in temperate forest soils. Ecology 92(4):892–902
Burns RG (1982) Enzyme-activity in soil—location and a possible role in microbial ecology. Soil Biol Biochem 14(5):423–427
Burns RG, Dick RP (2002) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, New York
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proc Natl Acad Sci USA 104:4990–4995
Chai SL, Tanner E (2010) Are we losing the best parts of our protected areas in tropical mountains? Biotropica 42:739–747
Clark D, Piper S, Keeling C, Clark D (2003) Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc Natl Acad Sci USA 100:5852–5857
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci USA 103(27):10316–10321
Cleveland CC, Townsend AR, Schimel D et al (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Biogeochemistry 13:623–645
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5(7):680–691
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87(2):492–503
Cleveland CC, Wieder WR, Reed SC, Townsend AR (2010) Experimental drought in a tropical rain forest increases soil carbon dioxide losses to the atmosphere. Ecology 91(8):2313–2323
Cleveland C, Townsend A, Taylor P et al (2011) Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol Lett 14:939–947
Crews T, Kitayama K, Fownes J et al (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–1424
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL, Team L (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Glob Change Biol 15(5):1339–1355
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92(3):621–632
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240
Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store—a shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 409(6817):149
Fujii K, Hartono A, Funakawa S et al (2011) Fluxes of dissolved organic carbon in three tropical secondary forests developed on serpentine and mudstone. Geoderma 163:119–126
Geisseler D, Horwath WR (2009) Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 53(1):87–98
German DP, Weintraub MN, Grandy AS, Lauber CL et al (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manag 148:185–206
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization and nitrification. In: Weaver RW (ed) Methods of soil analysis, part 2: microbiological and biochemical properties. Soil Science Society of America, Madison, pp 985–1018
Hedin LO, Brookshire E, Menge D, Barron A (2009) The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst 40:613–635
Herbert DA, Fownes JH (1995) Phosphorus limitation of forest leaf area and net primary production on a highly weathered soil. Biogeochemistry 29:223–235
Hernandez DL, Hobbie SE (2010) The effects of substrate composition, quantity, and diversity on microbial activity. Plant Soil 335(1–2):397–411
Houlton BZ, Sigman D, Hedin LO (2006) Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA 103:8745–8750
Ilstedt U, Singh S (2005) Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol (ultisol) compared with organic compost. Soil Biol Biochem 37:1407–1410
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647
Leff J, Nemergut D, Grandy A et al (2012a) The effects of soil bacterial community structure on decomposition in a tropical rain forest. Ecosystems 15:284–298
Leff J, Wieder W, Taylor P et al (2012b) Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest. Glob Change Biol 18:2969–2979
Lewis S, Lopez-Gonzalez G, Sonké B et al (2009) Increasing carbon storage in intact African tropical forests. Nature 457:1003–1006
Luo Y, Su B, Currie WS et al (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731
Malhi Y, Aragão L, Metcalfe DB et al (2009) Comprehensive assessment of carbon productivity, allocation and storage in three Amazonian forests. Glob Change Biol 15:1255–1274
Marklein A, Houlton B (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Martinelli L, Piccolo M, Townsend A et al (1999) Nitrogen stable isotopic composition of leaves and soil: tropical versus temperate forests. Biogeochemistry 46:45–65
Mcgill WB, Cole CV (1981) Comparative aspects of cycling of organic C, N, S and P through soil organic-matter. Geoderma 26(4):267–286
Miettinen J, Shi C, Liew SC (2012) Two decades of destruction in Southeast Asia’s peat swamp forests. Front Ecol Environ 10:124–128
Montano NM, García-Oliva F, Jaramillo VJ (2007) Dissolved organic carbon affects soil microbial activity and nitrogen dynamics in a Mexican tropical deciduous forest. Plant Soil 295:265–277
Moorhead DL, Sinsabaugh RL (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76(2):151–174
Murty D, Kirschbaum M, Mcmurtrie R, Mcgilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob Change Biol 8:105–123
Nardoto GB, Ometto JPHB, Ehleringer JR et al (2008) Understanding the influences of spatial patterns on N availability within the Brazilian amazon forest. Ecosystems 11:1234–1246
Nemani RR, Keeling CD, Hashimoto H et al (2003) Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 300:1560–1563
Nemergut DR, Cleveland CC, Wieder WR, Washenberger CL, Townsend AR (2010) Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biol Biochem 42(12):2153–2160
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49(2):175–190
Parton W, Silver WL, Burke IC et al (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315(5814):361–364
Pendall E, Bridgham S, Hanson PJ et al (2004) Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phytol 162:311–322
Posada JM, Schuur EAG (2011) Relationships among precipitation regime, nutrient availability, and carbon turnover in tropical rain forests. Oecologia 165:783–795
Randerson JT, Hoffman FM, Thornton PE et al (2009) Systematic assessment of terrestrial biogeochemistry in coupled climate-carbon models. Glob Change Biol 15:2462–2484
Reed SC, Cleveland CC, Townsend AR (2007) Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39:585–592
Reed SC, Vitousek PM, Cleveland CC (2010) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Reich PB, Hobbie SE, Lee T et al (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315
Sanchez PA, Bandy DE, Villachica JH, Nicholaides JJ (1982) Amazon basin soils: management for continuous crop production. Science 216:821–827
Sayer EJ, Joseph Wright S, Tanner EVJ et al (2012) Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 15:387–400
Silver WL, Lugo AE, Keller M (1999) Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 44(3):301–328
Sinsabaugh RL, Follstad Shah JJ (2012) Ecoenzymatic stoichiometry and ecological theory. Annu Rev Ecol Evol Syst 43:313–333
Sinsabaugh RL, Moorhead DL (1994) Resource-allocation to extracellular enzyme-production—a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26(10):1305–1311
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60(1):1–24
Sinsabaugh RL, Lauber C, Weintraub M et al (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462(7274):795–798
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Stursova M, Zifčáková L, Leigh MB et al (2012) Cellulose utilisation in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microb Ecol 80:735–746
Taylor PG (2012) Carbon and nutrient cycling in tropical forests: climatic, hydrologic and stoichiometric controls. Dissertation. University of Colorado, Boulder
Tiessen H, Moir JO (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. Lewis Publishers, Boca Raton, pp 75–86
Townsend A, Vitousek P, Holland E (1992) Tropical soils could dominate the short-term carbon cycle feedbacks to increased global temperatures. Clim Change 22:293–303
Townsend A, Asner G, Cleveland C (2008) The biogeochemical heterogeneity of tropical forests. Trends Ecol Evol 23:424–431
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82(4):946–954
Turner BL, Engelbrecht BMJ (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315
Turner B, Romero T (2010) Stability of hydrolytic enzyme activity and microbial phosphorus during storage of tropical rain forest soils. Soil Biol Biochem 42:459–465
Vitousek P, Matson P (1988) Nitrogen transformations in a range of tropical forest soils. Soil Biol Biochem 20:361–367
Vitousek PM, Sanford RJ (1986) Nutrient cycling in moist tropical forest. Annu Rev Ecol Syst 17:137–167
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36(9):1443–1451
Walker T, Syers J (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wallenstein MD, Weintraub MN (2008) Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol Biochem 40(9):2098–2106
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15(7):1631–1639
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90(12):3333–3341
Wieder WR, Cleveland CC, Townsend AR (2011) Throughfall exclusion and leaf litter addition drive higher rates of soil nitrous oxide emissions from a lowland wet tropical forest. Glob Change Biol. doi:10.1111/j.1365-2486.2011.02426.x
Wieder WR, Cleveland CC, Taylor PG et al (2012) Experimental removal and addition of leaf litter inputs reduces nitrate production and loss in a lowland tropical forest. Biogeochemistry. doi:10.1007/s10533-012-9793-1
Wood T, Lawrence D, Clark D, Chazdon R (2009) Rain forest nutrient cycling and productivity in response to large-scale litter manipulation. Ecology 90:109–121
Wright SJ (2005) Tropical forests in a changing environment. Trends Ecol Evol 20:553–560
Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF (2008) Simulated atmospheric NO3− deposition increases soil organic matter by slowing decomposition. Ecol Appl 18(8):2016–2027
Acknowledgments
We thank F. Campos Rivera, the Organización para Estudios Tropicales (OET) and the Ministerio de Ambiente, Energia y Telecommunicaciones (MINAET) for assisting with research permits and providing logistical support in Costa Rica, Marleny Jimenez and the Drake Bay Wilderness Camp for their generous access to field sites and W. Cambronero Castro for assistance in the field. We thank A. King and S. Schmidt for their support in conducting enzyme analyses. National Science Foundation Grants (DEB-0515744 and DEB-0852916) supported this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weintraub, S.R., Wieder, W.R., Cleveland, C.C. et al. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 114, 313–326 (2013). https://doi.org/10.1007/s10533-012-9812-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9812-2