Abstract
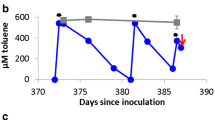
The study investigates two functional genes for toluene degradation across three redox conditions (nitrate and sulfate amended and methanogenic). The genes targeted include benzylsuccinate synthase α-subunit (bssA) and a gene recently identified as being a strong indicator of anaerobic aromatic degradation, called 6-oxocylcohex-1-ene-1-carbonyl-CoA hydrolase (bamA). In all, sixteen different anaerobic toluene degrading microcosms were investigated using several primers sets targeting bssA and one primer set targeting bamA. One bssA primer set (7772f/8546r) was the most successful in producing a strong amplicon (eight from sixteen) with the other bssA primers sets producing strong amplicons in six or less samples. In contrast, the bamA primer set (bam-sp9 and bam-asp1) produced a strong amplicon in DNA extracted from all except one microcosm. Partial bssA and bamA sequences were obtained for a number of samples and compared to those available in GenBank. The partial bssA sequences (from nitrate amended and methanogenic microcosms) were most similar to Thauera sp. DNT-1, Thauera aromatica, Aromatoleum aromaticum EbN1 and bssA clones from a study involving sulfate reducing toluene degradation. The bamA sequences obtained could be placed into five previously defined clades (bamA-clade 1, Georgfuchsia/Azoarcus, Magnetospirillum/Thauera Syntrophus and Geobacter clades), with the placement generally depending on redox conditions. Gene numbers were also correlated with toluene degradation and the final gene number for both genes differed considerably between the range of redox conditions. The work is the first in depth investigation of bamA diversity over a range of redox conditions and inoculum sources.





Similar content being viewed by others
References
Achong GR, Rodriguez AM, Spormann AM (2001) Benzylsuccinate synthase of Azoarcus sp. strain T: cloning, sequencing, transcriptional organization, and its role in anaerobic toluene and m-xylene mineralization. J Bacteriol 183(23):6763–6770
Agrawal A, Lal B (2009) Rapid detection and quantification of bisulfite reductase genes in oil field samples using real-time PCR. FEMS Microbiol Ecol 69(2):301–312
Beller HR, Kane SR, Legler TC, Alvarez PJJ (2002) A real-time polymerase chain reaction method for monitoring anaerobic, hydrotarbon-degrading bacteria based on a catabolic gene. Environ Sci Technol 36(18):3977–3984
Beller HR, Kane SR, Legler TC, McKelvie JR, Lollar BS, Pearson F, Balser L, MacKay DM (2008) Comparative assessments of benzene, toluene, and xylene natural attenuation by quantitative polymerase chain reaction analysis of a catabolic gene, signature metabolites, and compound-specific isotope analysis. Environ Sci Technol 42(16):6065–6072
Boll M (2005) Dearomatizing benzene ring reductases. J Mol Microbiol Biotechnol 10(2–4):132–142
Boll M, Fuchs G (1995) Benzoyl-coenzyme A reductase (dearomatizing), a key enzyme of anaerobic aromatic metabolism—ATP dependence of the reaction, purification and some properties of the enzyme from Thauera aromatica strain K172. Eur J Biochem 234(3):921–933
Botton S, van Harmelen M, Braster M, Parsons JR, Roling WFM (2007) Dominance of Geobacteraceae in BTX-degrading enrichments from an iron-reducing aquifer. FEMS Microbiol Ecol 62(1):118–130
Breese K, Boll M, Alt-Morbe J, Schagger H, Fuchs G (1998) Genes coding for the benzoyl-CoA pathway of anaerobic aromatic metabolism in the bacterium Thauera aromatica. Eur J Biochem 256(1):148–154
Fuchs G, Boll M, Heider J (2011) Microbial degradation of aromatic compounds—from one strategy to four. Nat Rev Microbiol 9(11):803–816
Jin YO, Mattes TE (2010) A quantitative PCR assay for aerobic, vinyl chloride- and ethene-assimilating microorganisms in groundwater. Environ Sci Technol 44(23):9036–9041
Jin S, Fallgren PH, Bilgin AA, Morris JM, Barnes PW (2007) Bioremediation of benzene, ethylbenzene, and xylenes in groundwater under iron-amended, sulfate-reducing conditions. Environ Toxicol Chem 26(2):249–253
Kane SR, Beller HR, Legler TC, Anderson RT (2002) Biochemical and genetic evidence of benzylsuccinate synthase in toluene-degrading, ferric iron-reducing Geobacter metallireducens. Biodegradation 13(2):149–154
Kuhner S, Wohlbrand L, Fritz I, Wruck W, Hultschig C, Hufnagel P, Kube M, Reinhardt R, Rabus R (2005) Substrate-dependent regulation of anaerobic degradation pathways for toluene and ethylbenzene in a denitrifying bacterium, strain EbN1. J Bacteriol 187(4):1493–1503
Kuntze K, Shinoda Y, Moutakki H, McInerney MJ, Vogt C, Richnow HH, Boll M (2008) 6-Oxocyclohex-1-ene-1-carbonyl-coenzyme A hydrolases from obligately anaerobic bacteria: characterization and identification of its gene as a functional marker for aromatic compounds degrading anaerobes. Environ Microbiol 10(6):1547–1556
Kuntze K, Vogt C, Richnow HH, Boll M (2011) Combined application of PCR-based functional assays for the detection of aromatic-compound-degrading anaerobes. Appl Environ Microbiol 77(14):5056–5061
Laempe D, Jahn M, Fuchs G (1999) 6-hydroxycyclohex-1-ene-1-carbonyl-CoA dehydrogenase and 6-oxocycsohex-1-ene-1-carbonyl-CoA hydrolase, enzymes of the benzoyl-CoA pathway of anaerobic aromatic metabolism in the denitrifying bacterium Thauera aromatica. Eur J Biochem 263(2):420–429
Li YN, Porter AW, Mumford A, Zhao XH, Young LV (2012) Bacterial community structure and bamA gene diversity in anaerobic degradation of toluene and benzoate under denitrifying conditions. J Appl Microbiol 112(2):269–279
Moore E (2009) Developing protocols to facilitate the enrichment and characterization of hydrocarbon-degrading anaerobic microbial communities. Department of Chemical Engineering and Applied Chemistry, MS Thesis, University of Toronto
Oka AR, Phelps CD, Zhu XY, Saber DL, Young LY (2011) Dual biomarkers of anaerobic hydrocarbon degradation in historically contaminated groundwater. Environ Sci Technol 45(8):3407–3414
Pereyra LP, Hiibel SR, Riquelme MVP, Reardon KF, Pruden A (2010) Detection and quantification of functional genes of cellulose-degrading, fermentative, and sulfate-reducing bacteria and methanogenic archaea. Appl Environ Microbiol 76(7):2192–2202
Pilloni G, von Netzer F, Engel M, Lueders T (2011) Electron acceptor-dependent identification of key anaerobic toluene degraders at a tar-oil-contaminated aquifer by Pyro-SIP. FEMS Microbiol Ecol 78(1):165–175
Song B, Ward BB (2005) Genetic diversity of benzoyl coenzyme A reductase genes detected in denitrifying isolates and estuarine sediment communities. Appl Environ Microbiol 71(4):2036–2045
Staats M, Braster M, Roling WFM (2011) Molecular diversity and distribution of aromatic hydrocarbon-degrading anaerobes across a landfill leachate plume. Environ Microbiol 13(5):1216–1227
Stelzer N, Buning C, Pfeifer F, Dohrmann AB, Tebbe CC, Nijenhuis I, Kastner M, Richnow HH (2006) In situ microcosms to evaluate natural attenuation potentials in contaminated aquifers. Org Geochem 37(10):1394–1410
Sun W, Cupples AM (2012) Diversity of five anaerobic toluene degrading microbial communities investigated using stable isotope probing (SIP). Appl Environ Microbiol 72(4):972–980
Washer CE, Edwards EA (2007) Identification and expression of benzylsuccinate synthase genes in a toluene-degrading methanogenic consortium. Appl Environ Microbiol 73(4):1367–1369
Winderl C, Schaefer S, Lueders T (2007) Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ Microbiol 9(4):1035–1046
Winderl C, Anneser B, Griebler C, Meckenstock RU, Lueders T (2008) Depth-resolved quantification of anaerobic toluene degraders and aquifer microbial community patterns in distinct redox zones of a tar oil contaminant plume. Appl Environ Microbiol 74(3):792–801
Winderl C, Penning H, von Netzer F, Meckenstock RU, Lueders T (2010) DNA-SIP identifies sulfate-reducing Clostridia as important toluene degraders in tar-oil-contaminated aquifer sediment. ISME J 4(10):1314–1325
Yang YR, Zeyer J (2003) Specific detection of Dehalococcoides species by fluorescence in situ hybridization with 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 69(5):2879–2883
Acknowledgments
Funding for this work was provided by a grant awarded to A. Cupples from the National Science Foundation (Grant 0853249). The authors thank Paul Fallgren (Western Research Institute), Kelvin Wong, Catherine Garnham, and Zhenbo Yue (Michigan State University) for supplying the contaminated soil sample (CSS), digester sludge (DSS), activated sludge (ASM) and anaerobic granular sludge (GSN) respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, W., Sun, X. & Cupples, A.M. Presence, diversity and enumeration of functional genes (bssA and bamA) relating to toluene degradation across a range of redox conditions and inoculum sources. Biodegradation 25, 189–203 (2014). https://doi.org/10.1007/s10532-013-9651-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-013-9651-4