Abstract
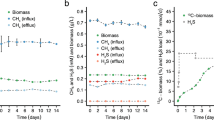
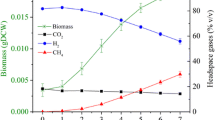
We develop a kinetic model that builds on the foundation of classic Monod kinetics, but incorporates new phenomena such as substrate thresholds and survival mode observed in experiments with the H2-oxidizing methanogen Methanobacterium bryantii M.o.H. We apply our model to the experimental data presented in our companion paper on H2 thresholds. The model accurately describes H2 consumption, CH4 generation, biomass growth, substrate thresholds, and survival state during batch experiments. Methane formation stops when its Gibbs free energy is equal zero, although this does not interrupt H2 oxidation. The thermodynamic threshold for H2 oxidation occurs when the free energy for oxidizing H2 and transferring electrons to biomass is no longer negative, at ∼0.4 nM. This threshold is not controlled by the Gibbs free energy equation of methanogenesis from H2 + HCO −3 as we show in our companion paper. Beyond this threshold, the microorganisms shift to a low-maintenance metabolism called “the survival state” in response to extended H2 starvation; adding the starvation response as another new feature of the kinetic model. A kinetic threshold (or S min), a natural feature of the Monod kinetics, is also captured by the model at H2 concentration of around ∼2,400 nM. S min is the minimum substrate concentration to maintain steady-state biomass concentration. Our model will be useful for interpreting threshold results and designing new studies to understand thresholds and their ecological implications.




Similar content being viewed by others
References
Brown DG, Komlos J, Jaffé P (2005) Simultaneous utilization of acetate and hydrogen by Geobacter sulfurreducen and implications for use of hydrogen as an indicator of redox conditions. Environ Sci Technol 39:3069–3076
Chapelle FH, Haack SK, Adriaens P, Henry MA, Bradley PM (1996) Comparison of Eh and H2 measurements for delineating redox processes in a contaminated aquifer. Environ Sci Technol 30:3565–3569
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO2, CH4, OCS, N2O, and NO). Microbiol Rev 60:609–640
Cord-Ruwisch R, Seitz H-J, Conrad R (1988) The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Arch Microbiol 149:350–357
Hoehler TM, Alperin MJ, Albert DB, Martens CS (1998) Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta 62:1745–1756
Hollocher TC (1982) The pathway of nitrogen and reduction enzymes of denitrification. Antonie Leewenhoek J Microbiol 150:319–326
Holliger C, Han D, Harmsen H, Ludwig W et al (1998) Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration. Arch Microbio 169:313–321
Karadagli F, Rittmann BE (2005) Kinetic characterization of Methanobacterium bryantii M.o.H. Environ Sci Technol 39:4900–4905
Karadagli F, Rittmann BE (2006, companion) Thermodynamic and kinetic analysis of the H2 threshold for methanobacterium bryantii M.o.H. Biodegradation (in press)
Kjelleberg S, Hermansson M, Marden P, Jones GW (1987) The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol 41:25–49
Kristjansson JK, Schönheit P, Thauer RK (1982) Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria. Arch Microbiol 131:278–282
Legall J, Fauque G (1988) Dissimilatory Reduction of sulfur compounds. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 469–585
Lovley DR (1985) Minimum threshold for hydrogen metabolism in methanogenic bacteria. Appl Environ Microbiol 49:1530–1531
Lovley DR, Goodwin ST (1988) Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim Cosmochim Acta 52:2993–3003
Namkung E, Rittmann BE (1987) Evaluation of bisubstrate secondary utilization kinetics by biofilms. Biotechnol Bioeng 29:335–342
Nicholls DG, Ferguson SJ (2002) Bioenergetics 3. Academic Press, London
Reeve AC, Bockman AT, Matin A (1984a) Role of protein degradation in the survival of carbon-starved Escherichia coli and Salmonella typhimurium. J Bacteriol 157:758–763
Reeve AC, Amy PS, Matin A (1984b) Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol 160:1041–1046
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, New York
Robinson JA, Tiedje JM (1984) Competition between sulfate-reducing and methanogenic bacteria for H2 under resting and growing conditions. Arch Microbiol 137:26–32
Smatlak CR, Gossett JR, Zinder SH (1996) Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ Sci Technol 30:2850–2858
Stumm W, Morgan JJ (1996) Aquatic Chemistry: Chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York
Stouthamer AH (1988) Dissimilatory reduction of oxidized nitrogen compounds. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York, pp 179–244
Siegele DA, Kolter R (1992) Life after Log. J Bacteriol 174:345–348
Thauer RK (1998) Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology 144:2377–2406
Valentine DL, Blanton DC, Reeburgh WS (2000) Hydrogen production by methanogens under low-hydrogen conditions. Arch Microbiol 174:415–421
Widdel F (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic organisms. Wiley, New York, pp 469–585
Yang Y, McCarty PL (1998) Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ Sci Technol 32:3591–3597
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karadagli, F., Rittmann, B.E. A mathematical model for the kinetics of Methanobacterium bryantii M.o.H. considering hydrogen thresholds. Biodegradation 18, 453–464 (2007). https://doi.org/10.1007/s10532-006-9078-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-006-9078-2