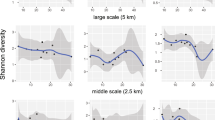
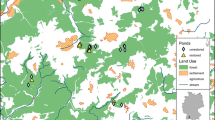
Abstract
Generally, dragonflies and damselflies (odonates) are considered aquatic invertebrates. However, the ecological requirements of their adults are not different from those of fully, terrestrial insects. Surprisingly, there is a very little information on whether the management and structure of surrounding habitats has any influence on the diversity and seasonal dynamics of odonates. This is important to know because recently, a large proportion of freshwater habitats in Central Europe have becomes surrounded by intensively managed habitats. The aim of this study was to investigate the effects of different types of terrestrial habitats on their long-term utilization by dragonflies and damselflies. I assumed that this pattern varied over time; therefore, I used generalized additive mixed models to analyze the effects of management on seasonal changes in the abundance of individuals in terrestrial environments. From my results, it was evident that the management practices of surrounding terrestrial habitats had a significant impact on the population dynamics of dragonflies. The abundance of dragonfly adults in surrounding terrestrial habitats increased toward the end of the season. However, this was only, when the natal aquatic habitat was not affected by fish farming and was able to supply surrounding terrestrial habitats with offspring. This was evidenced by the fact that, compared to areas with extensive water management, in sites with fish farming, seasonal increases in abundance was negligible. There is no doubt that the structure of surrounding terrestrial habitats has a significant influence on the diversity of terrestrial invertebrates. However, we must not forget that terrestrial habitats, regardless of their management, are not able to replace the poor quality of the aquatic (natal) habitat. Interestingly, the abundance of damselflies decreased toward the end of the season, regardless of the management practices of the surrounding areas. This indicates that their dynamics is more controlled by time stress or other similar mechanisms than that of dragonflies.






Similar content being viewed by others
References
Arlettaz R, Christe P, Lugon A et al (2001) Food availability dictates the timing of parturition in insectivorous mouse-eared bats. Oikos 95:105–111. doi:10.1034/j.1600-0706.2001.950112.x
Benke AC, Crowley PH, Johnson DM (1982) Interactions among coexisting larval Odonata: an in situ experiment using small enclosures. Hydrobiologia 94:121–130. doi:10.1007/BF00010890
Brodin T (2009) Behavioral syndrome over the boundaries of life—carryovers from larvae to adult damselfly. Behav Ecol 20:30–37. doi:10.1093/beheco/arn111
Bruppacher L, Pellet J, Arlettaz R, Humbert J-Y (2016) Simple modifications of mowing regime promote butterflies in extensively managed meadows: evidence from field-scale experiments. Biol Conserv 196:196–202. doi:10.1016/j.biocon.2016.02.018
Buri P, Arlettaz R, Humbert J-Y (2013) Delaying mowing and leaving uncut refuges boosts orthopterans in extensively managed meadows: evidence drawn from field-scale experimentation. Agric Ecosyst Environ 181:22–30. doi:10.1016/j.agee.2013.09.003
Bush A, Theischinger G, Nipperess D et al (2013) Dragonflies: climate canaries for river management. Divers Distrib 19:86–97. doi:10.1111/ddi.12007
Cizek O, Zamecnik J, Tropek R et al (2012) Diversification of mowing regime increases arthropods diversity in species-poor cultural hay meadows. J Insect Conserv 16:215–226. doi:10.1007/s10841-011-9407-6
Corbet PS (1999) Dragonflies: behaviour and ecology of Odonata. Harley Books, Colchester
Courtney GW, Cranston PS (2015) Diptera. In: Thorp J, Rogers C, Tockner K (eds) Thorp and covich’s freshwater invertebrates. Academic Press, Cambridge
Crumrine PW (2010) Body size, temperature, and seasonal differences in size structure influence the occurrence of cannibalism in larvae of the migratory dragonfly, Anax junius. Aquat Ecol 44:761–770. doi:10.1007/s10452-010-9314-z
De Block M, Stoks R (2004) Life-history variation in relation to time constraints in a damselfly. Oecologia 140:68–75. doi:10.1007/s00442-004-1575-6
De Block M, McPeek MA, Stoks R (2008a) Life-history evolution when Lestes damselflies invaded vernal ponds. Evolution 62:485–493. doi:10.1111/j.1558-5646.2007.00283.x
De Block M, McPeek MA, Stoks R (2008b) Life history plasticity to combined time and biotic constraints in Lestes damselflies from vernal and temporary ponds. Oikos 117:908–916. doi:10.1111/j.0030-1299.2008.16603.x
De Block M, Stoks R (2005) Fitness effects from egg to reproduction: bridging the life history transition. Ecology 86:185–197
Dolný A, Harabiš F, Bárta D et al (2013a) Aquatic insects indicate terrestrial habitat degradation: changes in taxonomical structure and functional diversity of dragonflies in tropical rainforest of East Kalimantan. Trop Zool 25:141–157. doi:10.1080/03946975.2012.717480
Dolný A, Mižičová H, Harabiš F (2013b) Natal philopatry in four European species of dragonflies (Odonata: Sympetrinae) and possible implications for conservation management. J Insect Conserv 17:821–829. doi:10.1007/s10841-013-9564-x
Dolný A, Harabiš F, Mižičová H (2014) Home range, movement, and distribution patterns of the threatened dragonfly Sympetrum depressiusculum (Odonata: Libellulidae): A thousand times greater territory to protect? PLoS ONE. doi:10.1371/journal.pone.0100408
Dolný A, Harabiš F, Bárta D (2016) Vážky (Insecta: Odonata) České republiky. Atlasy Academia, Praha
Foote LA, Rice Hornung CL (2005) Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecol Entomol 30:273–283. doi:10.1111/j.0307-6946.2005.00701.x
Harabiš F (2016) The value of terrestrial eco tones as refuges for winter damselflies (Odonata: Lestidae). J Insect Conserv 20:971–977. doi:10.1007/s10841-016-9929-z
Harabiš F, Dolný A (2011) The effect of ecological determinants on the dispersal abilities of Central European dragonflies (Odonata). Odonatologica 40:17–26
Hopper KR, Crowley PH, Kielman D (1996) Density dependence, hatching synchrony, and within-cohort cannibalism in young dragonfly larvae. Ecology 77:191–200. doi:10.2307/2265668
Hykel M, Harabiš F, Dolný A (2016) Assessment of the quality of the terrestrial habitat of the threatened dragonfly, Sympetrum depressiusculum (Odonata: Libellulidae). Eur J Entomol 113:476–481. doi:10.14411/eje.2016.062
Johansson F, Brodin T (2003) Effects of fish predators and abiotic factors on dragonfly community structure. J Freshw Ecol 18:415–423. doi:10.1080/02705060.2003.9663977
Johansson F, Suhling F (2004) Behaviour and growth of dragonfly larvae along a permanent to temporary water habitat gradient. Ecol Entomol 29:196–202. doi:10.1111/j.0307-6946.2004.00592.x
Kadoya T, Suda SI, Tsubaki Y, Washitani I (2008) The sensitivity of dragonflies to landscape structure differs between life-history groups. Landsc Ecol 23:149–158. doi:10.1007/s10980-007-9151-1
Kalkman VJ, Boudot J-P, Bernard R et al (2010) European red list of dragonflies. Publications Office of the European Union, Luxembourg
Knight TM, McCoy MW, Chase JM et al (2005) Trophic cascades across ecosystems. Nature 437:880–883. doi:10.1038/nature03962
McCauley SJ (2007) The role of local and regional processes in structuring larval dragonfly distributions across habitat gradients. Oikos 116:121–133. doi:10.1111/j.2006.0030-1299.15105.x
McCracken GF, Westbrook JK, Brown VA et al (2012) Bats track and exploit changes in insect pest populations. PLoS ONE. doi:10.1371/journal.pone.0043839
Oliver TH, Roy DB, Hill JK et al (2010) Heterogeneous landscapes promote population stability. Ecol Lett 13:473–484. doi:10.1111/j.1461-0248.2010.01441.x
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. doi:10.1093/bioinformatics/btg412
R Development Core Team (2015) R: a language and environment for statistical computing. The R foundation for statistical computing, Vienna, Austria. Available online at http://www.R-project.org/
Sahlén G, Ekestubbe K (2001) Identification of dragonflies (Odonata) as indicators of general species richness in boreal forest lakes. Biodivers Conserv 10:673–690. doi:10.1023/A:1016681524097
Sang A, Teder T (2011) Dragonflies cause spatial and temporal heterogeneity in habitat quality for butterflies. Insect Conserv Divers 4:257–264. doi:10.1111/j.1752-4598.2011.00134.x
Seifert LI, Scheu S (2012) Linking aquatic and terrestrial food webs—Odonata in boreal systems. Freshw Biol 57:1449–1457. doi:10.1111/j.1365-2427.2012.02807.x
Stoks R, Córdoba-Aguilar A (2012) Evolutionary ecology of Odonata: a complex life cycle perspective. Annu Rev Entomol 57:249–265. doi:10.1146/annurev-ento-120710-100557
Stoks R, Johansson F, De Block M (2008) Life-history plasticity under time stress in damselfly larvae. In: Córdoba-Aguilar A (ed) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, Oxford
Suhling F, Sahlén G, Kalkman V et al (2015) Odonata. In: Thorp J, Rogers C, Tockner K (eds) Thorp and covich’s freshwater invertebrates. Academic Press, Cambridge
Suhonen J, Rantala MJ, Honkavaara J (2008) Territoriality in odonates. In: Córdoba-Aguilar A (ed) Dragonflies and damselflies: model organisms for ecological and evolutionary research. Oxford University Press, Oxford
Termaat T, van Grunsven RHA, Plate CL, van Strien AJ (2015) Strong recovery of dragonflies in recent decades in The Netherlands. Freshw Sci 34:1094–1104. doi:10.1086/682669
Tews J, Brose U, Grimm V et al (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92. doi:10.1046/j.0305-0270.2003.00994.x
Tiitsaar A, Kaasik A, Teder T (2013) The effects of seasonally variable dragonfly predation on butterfly assemblages. Ecology 94:200–207. doi:10.1890/12-0541.1
Turchin P (2003) Complex population dynamics: a theoretical/empirical synthesis. Princeton University Press, Princeton
Wood SN (2006) Generalized additive models: an introduction with R. Chapman & Hall/CRC Press, Boca Raton
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B 73(1):3–36. doi:10.1111/j.1467-9868.2010.00749.x
Zahn A, Englmaier I, Drobny M (2010) Food availability for insectivores in grasslands—arthropod abundance in pastures, meadows and fallow land. Appl Ecol Environ Res 8:87–100
Acknowledgements
This research was part of a project EHP-CZ02-OV-1-024-2015 funded by the EEP grands and by IGA of Czech University of Life Sciences Prague (42900/1312/3166). I would like to thank Jana Hronková, Stanislav Švaček, Michal Hykel and Tereza Rusková for their assistance in collecting data and to 2 anonymous referees for their constructive and helpful comments on earlier versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Nigel E. Stork.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harabiš, F. Does the management of surrounding terrestrial habitats increase the tendency of odonates to leave aquatic habitats?. Biodivers Conserv 26, 2155–2167 (2017). https://doi.org/10.1007/s10531-017-1350-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-017-1350-8