Abstract
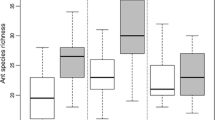
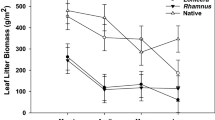
The Argentine ant, Linepithema humile (Mayr), is an invasive species that has been associated with various negative impacts in native communities around the world. These impacts, as for other invasive ants, are principally towards native ant species, and impacts on below-ground processes such as decomposition remain largely unexplored. We investigated the relationship between Argentine ants and invertebrate fauna, litter decomposition and soil microbial activity between paired invaded and uninvaded sites at two locations in Auckland, New Zealand, where there has been no research to date on their impacts. We examined the diversity and composition of invertebrate and microorganisms communities, and differences in soil and litter components. The composition of invertebrates (Order-level, ant and beetle species) was different between invaded and uninvaded sites, with fewer ants, isopods, amphipods, and fungus-feeding beetles at the invaded sites, whereas Collembola were more abundant at the invaded sites. There were significant differences in soil chemistry, including higher carbon and nitrogen microbial biomass at uninvaded sites. Several litter components were significantly different for Macropiper excelsum. The fibre content of litter was higher, and key nutrients (e.g. nitrogen) were lower, at invaded sites, indicating less breakdown of litter at invaded sites. A greater knowledge of the history of invasion at a site would clarify variation in the impacts of Argentine ants, but their persistence in the ground litter layer may have long-term implications for soil and plant health in native ecosystems.



Similar content being viewed by others
References
Allen R (1993) A permanent plot method for measuring changes in indigenous forests: a field manual. Landcare Research Ltd, Christchurch
Andersen AN (1997) Functional groups and patterns of organization in North American ant communities: a comparison with Australia. J Biogeogr 24:433–460
Andersen AN, Sparling GP (1997) Ants as indicators of restoration success: relationship with soil microbial biomass in the Australian seasonal tropics. Restor Ecol 5:109–114
Bending GD, Turner MK, Rayns F, Marxc MC, Wood M (2004) Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol Biochem 36:1785–1792
Berg B, Laskowski R (2006) Litter decomposition: a guide to carbon and nutrient turnover. Advances In ecological research 38. Academic Press, Burlington
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation, carbon sequestration, 2nd edn. Springer-Verlag, Berlin
Blancafort X, Gomez C (2005) Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol 28:49–55
Burton J, Chen C, Xu Z, Ghadiri H (2010) Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical. J Soil Sed 10:1267–1277
Cammeraat ELH, Risch AC (2008) The impact of ants on mineral soil properties and processes at different spatial scales. J Appl Entomol 132:285–294
Christian CE (2001) Consequences of a biological invasion reveal the importance of mutualism for plant communities. Nature 413:635–638
Clarke KR, Warwick RM (2005) Change in marine communities. An approach to statistical analysis and interpretation, 2nd edn. Plymouth Marine Laboratory, Plymouth
Cole FR, Medeiros AC, Loope LL, Zuehlke WW (1992) Effects of the Argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 73:1313–1322
Coleman DC, Crossley DA, Hendrix PF (2004) Fundamentals of soil ecology, 2nd edn. Elsevier Academic Press, San Diego
de Kock AE, Giliomee JH (1989) A survey of the Argentine ant, Iridomymrex humilis (Mayr), (Hymenoptera: Formicidae) in South African fynbos. J Entomol Soc S Afr 52:157–164
Don W (2007) Ants of New Zealand. Otago University Press, Dunedin
Dóstal P, Breznova M, Kozlickova V, Herben T, Kovar P (2005) Ant-induced soil modification and its effect on plant below-ground biomass. Pedobiologia 49:127–137
Duncan KW (1994) Terrestrial Talitridae (Crustacea: Amphipoda). Fauna of New Zealand series 31. Manaaki Whenua Press, Lincoln
Dunham AE, Mikheyev AS (2010) Influence of an invasive ant on grazing and detrital communities and nutrient fluxes in a tropical forest. Divers Distrib 16:33–42
Friend JA, Richardson AMM (1986) Biology of terrestrial amphipods. Annu Rev Entomol 31:25–28
Gotelli NJ, Arnett AE (2000) Biogeographic effects of red fire ant invasion. Ecol Lett 3:257–261
Green OL (1990) Entomologist sets new record at Mt Smart for Iridomyrmex humilis established in New Zealand. Weta 13:14–16
Green PT, Lake PS, O’Dowd DJ (1999) Monopolization of litter processing by a dominant land crab on a tropical oceanic island. Oecologia 119:435–444
Green PT, O’Dowd DJ, Lake PS (2008) Recruitment dynamics in a rainforest seedling community: context-independent impact of a keystone consumer. Oecologia 156:373–385
Hartley S, Harris R, Lester PJ (2006) Quantifying the uncertainty in the potential distribution of an invasive species: climate and the Argentine ant. Ecol Lett 9:1068–1079
Heller NE, Gordon DM (2006) Seasonal spatial dynamics and causes of nest movement in colonies of the invasive Argentine ant (Linepithema humile). Ecol Entomol 31:499–510
Hoffmann BD, Andersen AN, Hill GJE (1999) Impact of an introduced ant on native rain forest invertebrates: Pheidole megacephala in monsoonal Australia. Oecologia 120:595–604
Holway DA (1998) Effect of Argentine ant invasions on ground-dwelling arthropods in northern California riparian woodlands. Oecologia 116:252–258
Holway DA, Suarez AV (2006) Homogenization of ant communities in Mediterranean California: the effects of urbanization and invasion. Biol Conserv 127:319–326
Holway DA, Lach L, Suarez A, Tsutsui N, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Human KG, Gordon DM (1997) Effects of Argentine ants on invertebrate biodiversity in Northern California. Conserv Biol 11:1242–1248
Kenis M, Auger-Rozenberg MA, Roques A, Timms L, Pere C, Cock M, Settele J, Augustin S, Lopez-Vaamonde C (2009) Ecological effects of invasive alien insects. Biol Invasions 11:21–45
Krushelnycky PD, Gillespie RG (2010) Correlates of vulnerability among arthropod species threatened by invasive ants. Biodivers Conserv 19:1971–1988
Lafleur B, Hooper-Bu` LM, Mumma EP, Geaghan JP (2005) Soil fertility and plant growth in soils from pine forests and plantations: effect of invasive red imported fire ants Solenopsis invicta (Buren). Pedobiologia 49:415–423
Landcare Research (2008) Environmental chemistry laboratory tests. http://www.landcareresearch.co.nz/services/laboratories/eclab/eclabtest_list.asp. Accessed 28 July 2008
Lavelle E, Lattaud C, Trigo D, Barois I (1995) Mutualism and biodiversity in soils. Plant Soil 170:23–33
Leathwick J, Morgan F, Wilson G, Rutledge D, McLeod M, Johnston K (2003) Land environments of New Zealand technical guide. David Bateman Ltd, Auckland
McEwen WM (1987) Ecological districts and regions of New Zealand, 3rd ed. Biological Resources Centre Publication No 5. Department of Conservation, Wellington
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Moller H (1996) Lessons for invasion theory from social insects. Biol Conserv 78:125–142
Morrison LW (2002) Long-term impacts of an arthropod-community invasion by the imported fire ant, Solenopsis invicta. Ecology 83:2337–2345
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
O’Dowd DJ, Green PT, Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecol Lett 6:812–817
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–332
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Roura-Pascual N, Suarez AV, Gomez C, Pons P, Touyama Y, Wild AL, Peterson AT (2004) Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc R Soc Lond B 271(1557):2527–2535
Roura-Pascual N, Hui C, Ikeda T, Leday G, Richardson DM, Carpintero S, Espadaler X, Gómez C, Guénard B, Hartley S, Krushelnycky P, Lester PJ, McGeoch MA, Menke S, Pedersen J, Pitt J, Reyes J, Sanders NJ, Suarez AV, Touyama Y, Ward DF, Ward P, Worner SP (2011) The relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader. Proc Natl Acad Sci USA 108:220–225
Rowles AD, O’Dowd DJ (2009) Impacts of the invasive Argentine ant on native ants and other invertebrates in coastal scrub in south-eastern Australia. Austral Ecol 34:239–248
Rusek J (1998) Biodiversity of Collembola and their functional role in the ecosystem. Biodivers Conserv 7:1207–1219
Sanders NJ, Suarez AV (2011) Elton’s insights into the ecology of ant invasions: lessons learned and lessons still to be learned. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Blackwell, New York, pp 239–251
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive ant species. Proc Natl Acad Sci USA 100:2474–2477
Solaiman K (2007) Measurement of microbial biomass and activity in soil. In: Varman A, Oelmüller R (eds) Soil biology, vol 11., Advanced techniques in microbiologySpringer-Verlag, Berlin, pp 201–211
Standish RJ, Williams PA, Robertson AW, Scott NA, Hedderley DI (2004) Invasion by a perennial herb increases decomposition rate and alters nutrient availability in warm temperate lowland forest remnants. Biol Invasions 6:71–81
Suarez AV, Case TJ (2002) Bottom-up effects on persistence of a specialist predator: ant invasions and horned lizards. Ecol Appl 12:291–298
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal Southern California. Ecology 79:2041–2056
Suarez AV, Holway DA, Case TJ (2001) Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc Natl Acad Sci USA 98:1095–1100
Suarez AV, Yeh P, Case TJ (2005) Impacts of Argentine ants on avian nesting success. Insect Soc 52:378–382
Thomas ML, Holway DA (2005) Condition-specific competition between invasive Argentine ants and Australian Iridomymrex. J Anim Ecol 74:532–542
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evol 21:208–216
Waipara NW, Obanor F, Walter M (2002) Impact of phylloplane management on microbial populations. NZ Plant Prot 56:125–128
Walters AC (2006) Invasion by Argentine ants (Hymenoptera: Formicidae) in South Australia: impacts on community composition and abundance of invertebrates in urban parklands. Austral Ecol 31:567–576
Walters AC, Mackay DA (2003) The impact of the Argentine ant, Linepithema humile (Mayr), on native ants and other invertebrates in South Australia. Rec S Aust Mus 7:17–24
Ward DF (2009) The diversity, community composition and seasonality of native ants in northern New Zealand. Myrmecol News 12:195–200
Ward DF, Harris R (2005) Invasibility of native habitats by Argentine ants, Linepithema humile, in New Zealand. NZ J Ecol 29:215–219
Ward DF, New TR, Yen AL (2001) Effects of pitfall trap spacing on the abundance, richness and composition of invertebrate catches. J Insect Conserv 5:47–53
Ward DF, Harris RJ, Stanley MC (2005) Human-mediated range expansion of Argentine ants Linepithema humile (Hymenoptera: Formicidae) in New Zealand. Sociobiology 45:401–407
Ward DF, Harris RJ, Hartley S, Lester PJ, Stanley MC, Suckling DM, Toft RJ (2010) Twenty years of Argentine ants in New Zealand: past research and future priorities for applied management. NZ Entomol 33:67–78
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Wardle DA, Hyodo F, Bardgett RD, Yeates GW, Nilsson MC (2011) Long-term aboveground and belowground consequences of red wood ant exclusion in boreal forest. Ecology 92:645–656
Wider RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642
Williams DF (1994) Exotic ants: biology, impact, and control of introduced species. Westview Press, Boulder
Wootton JT (1994) The nature and consequences of indirect effects. Ann Rev Ecol Syst 25:443–466
Zelikova TJ, Sanders NJ, Dunn RR (2011) The mixed effects of experimental ant removal on seedling distribution, belowground invertebrates, and soil nutrients. Ecosphere 2(5):art63. doi:10.1890/ES11-00073.1
Acknowledgments
We thank staff at Auckland and North Shore city councils for permission to carry out this work; Robyn Simcock, Brian Daly, and Graham Sparling for advice on soil and chemical sampling; Chris Winks, Shane Hona, Josie Galbraith, Sarah Harrison, Sarah Knight for field assistance; Jo Rees for sorting invertebrate samples and Stephen Thorpe for identifying beetles; Nick Waipara and Olimpia Timudo for microbial advice, laboratory work and identification; and Guy Forrester for statistical advice; Richard Toft and Nathan Sanders for helpful comments on earlier versions of this manuscript. This work was supported by the Foundation for Research, Science and Technology (FRST C09X0507).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stanley, M.C., Ward, D.F. Impacts of Argentine ants on invertebrate communities with below-ground consequences. Biodivers Conserv 21, 2653–2669 (2012). https://doi.org/10.1007/s10531-012-0324-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0324-0