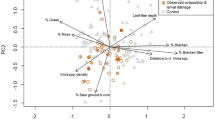
Abstract
Habitat quality and the impact of natural enemies might profoundly affect metapopulation dynamics and viability. However, their relative impact has usually been considered independently. Here we address the question of how caterpillar habitat quality and parasitism prevalence interact to shape habitat selection in the bog fritillary butterfly Boloria eunomia, parasitized at the caterpillar stage by a specialist wasp, Cotesia eunomiae. We first classified habitat quality by relating caterpillar density to descriptors of different microhabitat types. Second, we investigated parasitism prevalence in those different microhabitats. Our results show that caterpillars and parasitoids mapped onto the same microhabitats, mainly zones with high abundance of the host plant. Accordingly, we suggest that both egg-laying females and parasitoids use the same cues for habitat selection. As a consequence, there should be a fitness cost for B. eunomia females to lay their eggs in places where parasitism prevalence is high. We indeed detected that B. eunomia females frequently laid eggs in habitat types that presented suboptimal microhabitat conditions for caterpillars. This suggests that the lower parasitism prevalence in these suboptimal habitat types counterbalances lower caterpillar survival, leading to an overall similar survival in optimal and suboptimal habitat types. Spreading eggs in different habitat types is thus expected to be a safe strategy to mitigate the adverse possible effects of environmental stochasticity and parasitism prevalence on offspring survival unequal among microhabitat types. From a conservation viewpoint, the preservation of habitat heterogeneity, including optimal but also suboptimal habitat areas, is crucial to ensure persistence of both the host and its parasitoid.





Similar content being viewed by others
Abbreviations
- AICc:
-
Akaike’s information criterion corrected for small samples
- CMR:
-
Capture-Mark-Recapture
- DCA:
-
Detrended correspondence analysis
- HOST:
-
Abundance of the host plant Persicaria bistorta
- TOPO:
-
Microhabitat topography
- VEGE1:
-
First axis of a Detrended correspondence analysis summarizing plant species composition
- VEGE2:
-
Second axis of a Detrended correspondence analysis summarizing plant species composition
- CLIM1:
-
First axis of a Principal component analysis summarizing local microclimatic conditions
- CLIM2:
-
Second axis of a Principal component analysis summarizing local microclimatic conditions
References
Albanese G, Vickery PD, Sievert PR (2008) Microhabitat use by larvae and females of a rare barrens butterfly, frosted elfin (Callophrys irus). J Insect Conserv 12:603–615
Alonso C (1997) Choosing a place to grow. Importance of within-plant abiotic microenvironment for Yponomeuta mahalebella. Entomol Exp Appl 83:171–180
Anderson DR (2008) Model based inference in the life sciences. Springer, New York
Andow DA, Prokrym DR (1990) Plant structural complexity and host-finding by a parasitoid. Oecologia 82:162–165
Anthes N, Fartmann T, Hermann G, Kaule G (2003) Combining larval habitat quality and metapopulation structure-the key for successful management of pre-alpine Euphydryas aurinia colonies. J Insect Conserv 7:175–185
Bergerot B, Julliard R, Baguette M (2010) Metacommunity dynamics: decline of functional relationship along a habitat fragmentation gradient. PLoS One 5(6):e11294
Betzholtz PE, Ehrig A, Lindeborg M, Dinnetz P (2007) Food plant density, patch isolation and vegetation height determine occurrence in a Swedish metapopulation of the marsh fritillary Euphydryas aurinia (Rottemburg, 1775) (Lepidoptera, Nymphalidae). J Insect Conserv 11:343–350
Bezemer TM, Harvey JA, Kamp AFD et al (2010) Behaviour of male and female parasitoids in the field: influence of patch size, host density, and habitat complexity. Ecol Entomol 35:341–351
Dempster JP (1984) The natural enemies of butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies, 11th symposium of the Royal Entomological Society of London. Academic, London, pp 97–104
Dennis RLH, Shreeve TG, Van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
Dicke M, van Poecke RMP, de Boer JG (2003) Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl Ecol 4:27–42
Ellenberg H (1974) Zeigerwerte der gefässpflanzen mitteleuropas. Scripta geobotanica IX, Göttingen
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Ann Rev Ecol Syst 34:487–515
Ford HD, Ford EB (1930) Fluctuation in numbers and its influence on variation, in Melitaea aurinia, Rott. (Lepidoptera). Trans R Soc London 78:345–351
Godfray H (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, New Jersey
Gols R, Witjes LMA, Van Loon JJA et al (2008) The effect of direct and indirect defenses in two wild brassicaceous plant species on a specialist herbivore and its gregarious endoparasitoid. Entomol Exp Appl 128:99–108
Gotthard K (2008) Adaptive growth decisions in butterflies. Bioscience 58:222–230
Haddad NM, Crutsinger GM, Gross K et al (2009) Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol Lett 12:1029–1039
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Pakkala T, Kuussaari M, Lei GC (1995) Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 72:21–28
Heard SB, Stireman JO, Nason JD et al (2006) On the elusiveness of enemy-free space: spatial, temporal, and host-plant-related variation in parasitoid attack rates on three gall makers of goldenrods. Oecologia 150:421–434
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Kankare M, van Nouhuys S, Gaggiotti O, Hanski I (2005) Metapopulation genetic structure of two coexisting parasitoids of the Glanville fritillary butterfly. Oecologia 143:77–84
Klapwijk MJ, Grobler BC, Ward K, Wheeler D, Lewis OT (2010) Influence of experimental warming and shading on host-parasitoid synchrony. Global Change Biol 16:102–112
Konvicka M, Hula V, Fric Z (2003) Habitat of pre-hibernating larvae of the endangered butterfly Euphydryas aurinia (Lepidoptera: Nymphalidae): what can be learned from vegetation composition and architecture? Eur J Entomol 100:313–322
Kuhrt U, Samietz J, Dorn S (2005) Thermoregulation behaviour in codling moth larvae. Physiol Entomol 30:54–61
Lei GC, Hanski I (1997) Metapopulation structure of Cotesia melitaearum, a specialist parasitoid of the butterfly Melitaea cinxia. Oikos 78:91–100
Lei GC, Hanski I (1998) Spatial dynamics of two competing specialist parasitoids in a host metapopulation. J Anim Ecol 67:422–433
Liebhold A, Koenig WD, Bjornstad ON (2004) Spatial synchrony in population dynamics. Ann Rev Ecol Syst 35:467–490
Lill JT, Marquis RJ, Ricklefs RE (2002) Host plants influence parasitism of forest caterpillars. Nature 417:170–173
Obermaier E, Heisswolf A, Poethke HJ, Randlkofer B, Meiners T (2008) Plant architecture and vegetation structure: two ways for insect herbivores to escape parasitism. Eur J Entomol 105:233–240
Ohsaki N, Sato Y (1994) Food plant choice of Pieris butterflies as a trade-off between parasitoid avoidance and quality of plants. Ecology 75:59–68
Penuelas J, Filella I, Stefanescu C, Llusia J (2005) Caterpillars of Euphydryas aurinia (Lepidoptera:Nymphalidae) feeding on Succisa pratensis leaves induce large foliar emissions of methanol. New Phytol 167:851–857
Randlkofer B, Obermaier E, Meiners T (2007) Mother’s choice of the oviposition site: balancing risk of egg parasitism and need of food supply for the progeny with an infochemical shelter? Chemoecology 17:177–186
Roy DB, Thomas JA (2003) Seasonal variation in the niche, habitat availability and population fluctuations of a bivoltine thermophilous insect near its range margin. Oecologia 134:439–444
Schtickzelle N, Baguette M (2004) Metapopulation viability analysis of the bog fritillary butterfly using RAMAS/GIS. Oikos 104:277–290
Schtickzelle N, Mennechez G, Baguette M (2006) Dispersal depression with habitat fragmentation in the bog fritillary butterfly. Ecology 87:1057–1065
Schtickzelle N, Joiris A, Van Dyck H, Baguette M (2007a) Quantitative analysis of changes in movement behaviour within and outside habitat in a specialist butterfly. BMC Evol Biol 7:4
Schtickzelle N, Turlure C, Baguette M (2007b) Grazing management impacts on the viability of the threatened bog fritillary butterfly Proclossiana eunomia. Biol Conserv 136:651–660
Shaw MR (2006) Habitat considerations for parasitic wasps (Hymenoptera). J Insect Conserv 10:117–127
Shaw MR (2009) Cotesia Cameron (Hymenoptera: Braconidae: Microgastrinae) parasitoids if Heliconiinae (Lepidoptera: Nymphalidae) in Europe, with description of three new species. Br J Entomol Nat His 22:133–146
Shaw MR, Stefanescu C, van Nouhuys S (2009) Parasitism of European butterflies (hesperioidea and papilionoidea). In: Settele J, Shreeve TG, Konvicka M, Van Dyck H (eds) Ecology of butterflies in Europe. Cambridge University Press, Cambridge, pp 130–156
Steiner S, Steidle JLM, Ruther J (2007) Host-associated kairomones used for habitat orientation in the parasitoid Lariophagus distinguendus (Hymenoptera: Pteromalidae). J Stored Prod Res 43:587–593
Sutcliffe OL, Thomas CD, Moss D (1996) Spatial synchrony and asynchrony in butterfly population dynamics. J Anim Ecol 65:85–95
Ter Braak CJF, Smilauer P (2002) Canoco reference manual and canodraw for windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, USA
Thomas JA, Bourn NAD, Clarke RT et al (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc R Soc London Ser B 268:1791–1796
Turlure C (2009) Habitat from a butterfly’s point of view: how specialist butterflies map onto ecological resources. PhD thesis, Biodiversity Research Centre, Université catholique de Louvain, Louvain-la-Neuve
Turlure C, Van Dyck H, Schtickzelle N, Baguette M (2009) Resource-based habitat definition, niche overlap and conservation of two sympatric glacial relict butterflies. Oikos 118:950–960
Turlure C, Baguette M, Stevens V, Maes D (2011a) Species- and sex-specific adjustments of movement behavior to landscape heterogeneity in butterflies. Behav Ecol 22:967–975
Turlure C, Radchuk V, Baguette M, Van Dyck H, Schtickzelle N (2011b) On the significance of structural vegetation elements for caterpillar thermoregulation in two peat bog butterflies: Boloria eunomia and B. aquilonaris. J Therm Biol 36:173–180
Tylianakis JM, Tscharntke T, Lewis OT (2007) Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445:202–205
Umbanhowar J, Maron J, Harrison S (2003) Density-dependent foraging behaviors in a parasitoid lead to density-dependent parasitism of its host. Oecologia 137:123–130
Van Alpen JJM, Bernstein C, Driessen G (2003) Information acquisition and time allocation in insect parasitoids. Trends Ecol Evol 18:81–87
Van der Putten WH, De Ruiter PC, Bezemer TM et al (2004) Trophic interactions in a changing world. Basic Appl Ecol 5:487–494
Van Nouhuys S, Hanski I (2002) Multitrophic interactions in space: metacommunity dynamics in fragmented landscapes. In: Tscharntke T, Hawkins BA (eds) Multiple level interactions. Cambridge University Press, Cambridge, pp 124–147
Vidal S, Tscharntke T (2001) Special feature: Multitrophic plant-insect interactions. Basic Appl Ecol 2:1–2
Vinson SB, Pennacchio F, Lanzrein B (1998) Interactions between parasitoids and their hosts: An introduction and perspective. J Insect Physiol 44:701–702
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
Acknowledgments
J.C. is teaching assistant at the UCL. N.S. is Research Associate from the Fund for Scientific Research-FNRS and acknowledges its financial support. Special capture licenses for Boloria eunomia and site access were provided by the “Ministère de la Région Wallonne”. This paper is contribution BRC228 of the Biodiversity Research Centre at UCL. MB and CT acknowledged supports from the project TenLamas funded by the French Agence Nationale de la Recherche (ANR) through the EU FP6 BiodivERsA Eranet, and the EU FP7 SCALES project (“Securing the conservations of biodiversity across Administrative levels and spatial, temporal and Ecological Scales”; project no. 226852).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choutt, J., Turlure, C., Baguette, M. et al. Parasitism cost of living in a high quality habitat in the bog fritillary butterfly. Biodivers Conserv 20, 3117–3131 (2011). https://doi.org/10.1007/s10531-011-0151-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-011-0151-8