Abstract
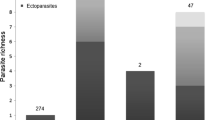
Relationships between parasitism and invasion success are increasingly evidenced in the literature. However, the dynamic nature of the major parasite-related processes has been rarely taken into account until now, while the residence time of invaders in colonized regions was shown to be associated to crucial changes in parasite communities. Here, we strive to bridge this gap using a temporal survey of rodent populations along one invasion route of the exotic house mouse Mus musculus domesticus that currently invades North Senegal. In this study, we investigated whether gastrointestinal helminth (GIH) assemblages changed over time in native (Mastomys erythroleucus) and/or invasive (M. m. domesticus) rodent populations sampled at an invasion front, and whether these potential changes may be associated to the invasion success of the exotic mouse. Four years separated two rodent sampling campaigns (2013 and 2016/17) in six localities. Despite being relatively short, the timeframe considered here allowed to evidence significant patterns in rodent communities and their GIH assemblages. At the host community level, we showed that the exotic mouse was now established at all sites, becoming the dominant species in sites where it was not recorded before. At the GIH community level, increased infection of the single shared cestode (Mathevotaenia symmetrica) in both rodent species brought support to the “spill-back” hypothesis. Infection levels of GIH that remained low at the invasion front in invading mice over time also supported the “enemy release” hypothesis. Both hypotheses should deserve further experimental work to demonstrate their role in the invasion success of the house mouse in Senegal.


Similar content being viewed by others
References
Anya AO (1966) Studies on the biology of some oxyurid nematodes II The hatching of eggs, development of Aspiculuris tetraptera Schulz, within the host. J Helminthol 40(3–4):261–268
Barton K, Barton MK (2019) Package ‘MuMIn’ Multi-model inference version, R package version 1436. https://R-Forge.R-project.org/projects/mumin
Behnke JM, Bajer A, Harris PD, Newington L, Pidgeon E, Rowlands G, Sheriff C, Kulis-Malkowska K, Sinski E, Gilbert FS, Barnard CJ (2008) Temporal, between-site variation in helminth communities of bank voles (Myodes glareolus) from NE Poland 1 Regional fauna, component community levels. Parasitology 135:985–997
Behnke JM, Eira C, Rogan M, Gilbert FS, Torres J, Miquel J, Lewis JW (2009) Helminth species richness in wild wood mice, Apodemus sylvaticus, is enhanced by the presence of the intestinal nematode Heligmosomoides polygyrus. Parasitology 136(7):793–804
Behnke JM, Stewart A, Bajer A, Grzybek M, Harris PD et al (2015) Bank voles (Myodes glareolus), house mice (Mus musculus musculus; M m domesticus) in Europe are each parasitized by their own distinct species of Aspiculuris (Nematoda, Oxyurida). Parasitology 142(12):1493–1505
Bell SS, White A, Sherratt JA, Boots M (2009) Invading with biological weapons: the role of shared disease in ecological invasion. Theoret Ecol 2:53–66
Beveridge I (2008) Mathevotaenia niuguiniensis n sp (Cestoda: Anoplocephalidae: Linstowiinae) from the water-rat Parahydromys asper (Thomas) in Papua New Guinea, with a list of species of Mathevotaenia Akumyan, 1946. Syst Parasitol 71(3):189
Blossey B, Nötzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889
Brouat C, Kane M, Diouf M, Ba K, Sall-Dramé R, Duplantier JM (2007) Host ecology, variation in helminth community structure in Mastomys rodents from Senegal. Parasitology 134(3):437–450
Blackburn TM, Ewen JG (2017) Parasites as drivers, passengers of human-mediated biological invasions. EcoHealth 14(1):61–73
Castañeda I, Pisanu B, Díaz M, Rézouki C, Baudry E, Chapuis JL, Bonnaud E (2018) Minimising trapping effort without affecting population density estimations for small mammals. Mamm Biol 93(1):144–152
Carolus H, Muzarabani KC, Hammoud C, Schols R, Volckaert FA, Barson M, Huyse T (2019) A cascade of biological invasions, parasite spillback in man-made Lake Kariba. Sci Total Environ 659:1283–1292
Chalkowski K, Lepczyk CA, Zohdy S (2018) Parasite ecology of invasive species: conceptual framework new hypotheses. Trends Parasitol 34(8):655–663
Christe P, Morand S, Michaux J (2006) Biological conservation, parasitism. In: Morand S, Krasnov BR, Poulin R (eds) Micromammals macroparasites. Springer, Tokyo, pp 593–613
Coates A, Barnett LK, Hoskin C, Phillips BL (2017) Living on the edge: parasite prevalence changes dramatically across a range edge in an invasive gecko. Am Nat 189(2):178–183
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7(8):721–733
Cornet S, Brouat C, Diagne C, Charbonnel N (2016) Eco-immunology, bioinvasion: revisiting the evolution of increased competitive ability hypotheses. Evol Appl 9(8):952–962
Costa APL, Takemoto RM, Vitule JRS (2018) Metazoan parasites of Micropterus salmoides (Lacépède 1802)(Perciformes, Centrarchidae): a review with evidences of spillover, spillback. Parasitol Res 117(6):1671–1681
De Boeck P, Bakker M, Zwitser R, Nivard M, Hofman A, Tuerlinckx F, Partchev I (2011) The estimation of item response models with the lmer function from the lme4 package in R. J Stat Softw 39(12):1–28
de Villemereuil P, Gaggiotti OE, Mouterde M, Till-Bottraud I (2016) Common garden experiments in the genomic era: new perspectives and opportunities. Heredity 116(3):249–254
Diagne C, Ribas A, Charbonnel N, Dalecky A, Tatard C, Gauthier P, Niang Y (2016) Parasites, invasions: changes in gastrointestinal helminth assemblages in invasive, native rodents in Senegal. Int J Parasitol 46(13–14):857–869
Dalecky A, Bâ K, Piry S, Lippens C, Diagne CA, Kane M, Sarr N (2015) Range expansion of the invasive house mouse Mus musculus domesticus in Senegal, West Africa: a synthesis of trapping data over three decades, 1983–2014. Mamm Rev 45(3):176–190
Deter J, Cosson JF, Chaval Y, Charbonnel N, Morand S (2007) The intestinal nematode Trichuris arvicolae affects the fecundity of its host, the common vole Microtus arvalis. Parasitol Res 101:1161–1164
Dunn AM, Hatcher MJ (2015) Parasites, biological invasions: parallels, interactions, control. Trends Parasitol 31(5):189–199
Dunn AM, Torchin ME, Hatcher MJ, Kotanen PM, Blumenthal DM, Byers JE, Kanarek AR (2012) Indirect effects of parasites in invasions. Funct Ecol 26(6):1262–1274
Ezenwa VO (2016) Helminth–microparasite co-infection in wildlife: lessons from ruminants, rodents, rabbits. Parasite Immunol 38(9):527–534
Gagne RB, Blum MJ (2016) Parasitism of a native H awaiian stream fish by an introduced nematode increases with declining precipitation across a natural rainfall gradient. Ecol Freshwater Fish 25(3):476–486
Gendron AD, Marcogliese DJ (2016) Reduced survival of a native parasite in the invasive round goby: evidence for the dilution hypothesis? Aquat Invasions 11(2):189–198
Gendron AD, Marcogliese DJ, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St Lawrence Basin. Biol invasions 14:367–384
Goedknegt MA, Schuster AK, Buschbaum C, Gergs R, Jung AS, Luttikhuizen PC, Thieltges DW (2017) Spillover but no spillback of two invasive parasitic copepods from invasive Pacific oysters (Crassostrea gigas) to native bivalve hosts. Biol Invasions 19(1):365–379
Gossner MM, Chao A, Bailey RI, Prinzing A (2009) Native fauna on exotic trees: phylogenetic conservatism, geographic contingency in two lineages of phytophages on two lineages of trees. Am Nat 173:599–614
Graham AL (2008) Ecological rules governing helminthemicroparasite coinfection. Proc Nat Acad Sci 105:566e570
Granjon L, Duplantier JM (2009) Les rongeurs de l’Afrique sahélo-soudanienne Publications scientifiques du Muséum. Marseille, France
Hajek AE, Tobin PC (2011) Introduced pathogens follow the invasion front of a spreading alien host. J Animal Ecol 80(6):1217–1226
Hatcher MJ, Dick JT, Dunn AM (2012) Disease emergence, invasions. Funct Ecol 26(6):1275–1287
Hawkes CV (2007) Are invaders moving targets? The generality, persistence of advantages in size, reproduction, enemy release in invasive plants species with time since introduction. Am Nat 170:832–843
Jones CM, Brown MJF (2014) Parasites, genetic diversity in an invasive bumblebee. J Animal Ecol 83(6): 1428–1440
Johnson PTJ, Thieltges DW (2010) Diversity, decoys, the dilution effect: how ecological communities affect disease risk. J Exp Biol 213(6):961–970
Julius RS, Schwan EV, Chimimba CT (2018) Molecular characterization of cosmopolitan, potentially co-invasive helminths of commensal, murid rodents in Gauteng Province South Africa. Parasitol Res 117(6):1729–1736
Kalyankar SD, Deshmukh A (1980) A report of the cestode species Mathevotaenia symmetrica Akumyan, 1946 (Cestoda: Anoplocephalidae) from a wild-rat Mus musculus in Maharashtra. Bioresearch 4(2):49–50
Keane RM, Crawley MJ (2002) Exotic plant invasions, the enemy release hypothesis. Trends Ecol Evol 17:164–170
Keesing F, Holt RD, Ostfeld RS (2006) Effects of species diversity on disease risk. Ecol Lett 9(4):485–498
Kelly DW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology 90(8):2047–2056
Kołodziej-Sobocińska M, Brzeziński M, Niemczynowicz A, Zalewski A (2018) High parasite infection level in non-native invasive species: it is just a matter of time. Ecography 41(8):1283–1294
Krakau M, Thieltges DW, Reise K (2006) Native parasites adopt introduced bivalves of the North Sea. Biol Invasions 8:919–925
Kreisinger J, Bastien G, Hauffe HC, Marchesi J, Perkins SE (2015) Interactions between multiple helminths, the gut microbiota in wild rodents. Philos R Soc B 370(1675):20140295
Lafferty KD, Torchin ME, Kuris AM (2010) The geography of host and parasite invasions. In: Morand S, Krasnov BR (eds) The biogeography of host–parasite interactions. Oxford University Press, New York, pp 191–203
Landaeta-Aqueveque C, del Rosario Robles M, Henríquez A, Yáñez-Meza A, Correa JP, González-Acuña D, Cattan PE (2018) Phylogenetic, ecological factors affecting the sharing of helminths between native, introduced rodents in Central Chile. Parasitology 145(12):1570–1576
Leggett HC, Wild G, West SA, Buckling A (2017) Fast-killing parasites can be favoured in spatially structured populations. Philos R Soc B 372(1719):20160096
Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ (2004) Competition, mutualism among the gut helminths of a mammalian host. Nature 428:840e844
Liebhold A, Bascompte J (2003) The Allee effect, stochastic dynamics, the eradication of alien species. Ecol lett 6:133–140
Lippens C, Estoup A, Hima MK, Loiseau A, Tatard C, Dalecky A, Niang Y (2017) Genetic structure, invasion history of the house mouse (Mus musculus domesticus) in Senegal West Africa: a legacy of colonial, contemporary times. Heredity 119(2):64
Loxton KC, Lawton C, Stafford P, Holland CV (2017) Parasite dynamics in an invaded ecosystem: helminth communities of native wood mice are impacted by the invasive bank vole. Parasitology 144(11):1476–1489
Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasit Parasit Wildl 3(2):171–177
MacLeod CJ, Paterson AM, Tompkins DM, Duncan RP (2010) Parasites lost–do invaders miss the boat or drown on arrival? Ecol lett 13(4):516–527
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis, graphics of microbiome census data. PLoS ONE 8(4):e61217
Mastitsky SE, Veres JK (2010) Field evidence for a parasite spillback caused by exotic mollusc Dreissena polymorpha in an invaded lake. Parasitol Res 106(3):667–675
Mitchell CE, Blumenthal V, Jarosˇı´k, EE, Puckett P, Pysek D (2010) Controls on pathogen species richness in plants’ introduced, native ranges: roles of residence time, range size, host traits. Ecol Lett 13:1525–1535
Miura O (2007) Molecular genetic approaches to elucidate the ecological, evolutionary issues associated with biological invasions. Ecol Res 22(6):876–883
Murai E (1974) Mathevotaenia symmetrica occidentalis ssp n (Cestoda, Linstowiidae) from rodents in Hungary. Parasitologia Hungarica 7:143–150
Nachman MW, Searle JB (1995) Why is the house mouse karyotype so variable? Trends Ecol Evol 10(10):397–402
Oksanen J, Blanchet FG, Kindt R, Legendre P, O’hara RB, Simpson GL, Wagner H (2010) Vegan: community ecology package R package version 117-4. https://cran.r-project.org
Paterson RA, Townsend CR, Tompkins DM, Poulin R (2012) Ecological determinants of parasite acquisition by exotic fish species. Oikos 121(11):1889–1895
Perkins SE, White TA, Pascoe EL, Gillingham EL (2017) Parasite community dynamics in an invasive vole–from focal introduction to wave front. Int J Parasit Parasit Wildl 6(3):412–419
Phillips BL, Kelehear C, Pizzatto L, Brown GP, Barton D, Shine R (2010) Parasites, pathogens lag behind their host during periods of host range advance. Ecology 91(3):872–881
Platvoet D, Rigaud T (2007) No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos 116(11): 1941–1953
Poulin R, Krasnov BR, Mouillot D, Thieltges DW (2011) The comparative ecology, biogeography of parasites. Philos Trans R Soc B 366:2379–2390
Prenter J, MacNeil C, Dick JT, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19(7):385–390
Prior KM, Hellmann JJ (2013) Does enemy loss cause release? A biogeographical comparison of parasitoid effects on an introduced insect. Ecology 94(5):1015–1024
Romeo C, Wauters LA, Ferrari N, Lanfranchi P, Martinoli A, Pisanu B, Saino N (2014) Macroparasite fauna of alien grey squirrels (Sciurus carolinensis): composition, variability, implications for native species. PLoS ONE 9(2):e88002
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86(2):228–233
Roy HE, Handley LJL, Schönrogge K, Poland RL, Purse BV (2011) Can the enemy release hypothesis explain the success of invasive alien predators, parasitoids? Biocontrol 56(4):451–468
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, McCauley DE (2001) The population biology of invasive species. Ann Rev Ecol Syst 32(1):305–332
Schultheis EH, Berardi AE, Lau JA (2015) No release for the wicked: enemy release is dynamic, not associated with invasiveness. Ecology 96(9):2446–2457
Sheath DJ, Williams CF, Reading AJ, Britton JR (2015) Parasites of non-native freshwater fishes introduced into England Wales suggest enemy release, parasite acquisition. Biol Invasions 17(8):2235–2246
Sikes RS, Gannon WL (2011) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mamm 92(1):235–253
Smith KF, Carpenter SM (2006) Potential spread of introduced black rat (Rattus rattus) parasites to endemic deer mice (Peromyscus maniculatus) on the California Channel Islands. Div Distrib 12(6):742–748
Spickett A, Junker K, Froeschke G, Haukisalmi V, Matthee S (2019) Nematodes, cestodes of rodents in South Africa: baseline data on diversity, geographic distribution. J Helminthol 94:1–17
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26(6):1249–1261
Taraschewski H (2006) Hosts, parasites as aliens. Helminthologia 80:99–128
Team R C (2015) R: A language, environment for statistical computing; 2015
Taffs LF (1976) Pinworm infections in laboratory rodents: a review. Lab Animals 10(1):1–13
Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M (2010) Species interactions in a parasite community drive infection risk in a wildlife population. Science 330:243e246
Telfer S, Bown K (1288e) The effects of invasion on parasite dynamics, communities. Funct Ecol 26:1288e1299
Tompkins DM, Sainsbury AW, Nettleton P, Buxton D, Gurnell J (2002) Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc R Soc B 269(1490):529–533
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species, their missing parasites. Nature 421:628–630
Tuttle LJ, Sikkel PC, Cure K, Hixon MA (2017) Parasite-mediated enemy release, low biotic resistance may facilitate invasion of Atlantic coral reefs by Pacific red lionfish (Pterois volitans). Biol Invasions 19(2):563–575
Vandegrift KJ, Hudson PJ (2009) Could parasites destabilize mouse populations? The potential role of Pterygodermatites peromysci in the population dynamics of free-living mice, Peromyscus leucopus. Int J Parasitol 39:1253–1262
Wattier RA, Haine ER, Beguet J, Martin G, Bollache L, Muskó IB, Rigaud T (2007) No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos 116(11):1941–1953
Young HS, Parker IM, Gilbert GS, Guerra AS, Nunn CL (2017) Introduced species, disease ecology, biodiversity–disease relationships. Trends Ecol Evol 32(1):41–54
Acknowledgements
We thank Khalilou Bâ, Aliou Sow, Moussa Sall, Jean Le Fur and Mamoudou Diallo for their help in collecting rodents. We thank all the people in Senegal who welcomed us into their homes for the purposes of small mammal trapping, as well as the Senegalese Head Office of Waters and Forests for allowing us to do fieldwork and to export samples. We are also grateful to Alexis Ribas for his precious advices and help in helminth identification. Samples are preserved in the CBGP—Small mammal collection (https://doi.org/10.15454/WWNUPO) and referenced in the associated Small Mammal Database. We thank Nathalie Sarr for data capture. Molecular data necessary for helminth identification were generated at the molecular biology platform of the CBGP.
Funding
This work was supported by the ENEMI project (funded by the Agence Nationale de la Recherche, ANR-11-JSV7-0006), the CERISE project (funded by the Fonds Français pour l’Environnement Mondial via the Fondation pour la Recherche sur la Biodiversité: AAP-SCEN-20B III), and the Labex DRIIHM, French programme "Investissements d'Avenir" (ANR-11-LABX-0010) managed by the Agence Nationale pour la Recherche (ANR). The French Research Institute for Development (IRD) provided the funding for a post-doctoral position.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Prior explicit agreement from relevant local authorities and individual owners was systematically obtained for each sampling campaign within private dwellings. Trapping sessions and transfer of biological samples were carried out in accordance with requirements of Senegalese and French legislations. Every realized protocol received explicit approval from the relevant institutional comitee (Centre de Biologie pour la Gestion des Populations (CBGP): Agrément pour l’utilisation d’animaux à des fins scientifiques D-34-169-1). All animal-related procedures were performed according to official ethical guidelines provided by the American Society of Mammalogists (Sikes and Gannon 2011). None of the rodent species investigated here has any protected status from the International Union for Conservation of Nature.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diagne, C., Granjon, L., Gueye, M.S. et al. Association between temporal patterns in helminth assemblages and successful range expansion of exotic Mus musculus domesticus in Senegal. Biol Invasions 22, 3003–3016 (2020). https://doi.org/10.1007/s10530-020-02304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02304-7