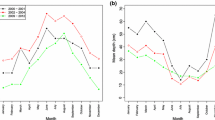
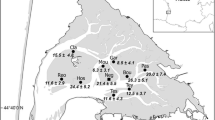
Abstract
The enemy release hypothesis postulates that non-native species establish and become abundant because coevolved enemies from the native range are missing or greatly reduced in the introduced range. We assessed whether the invasive New Zealand mud snail, Potamopyrgus antipodarum, is released from castrating trematode parasites by comparing prevalence and diversity of trematode parasites in P. antipodarum and co-occurring native snails in the western United States. Consistent with the enemy release hypothesis (1) P. antipodarum was not infected by trematodes at 80% of the sites and, relative to native snails, had low prevalence (4–5%) at the remaining sites, (2) across all sites, mean prevalence of trematodes was nine times lower in P. antipodarum than in co-occurring native snails and, (3) P. antipodarum were infected by half or fewer of the trematode taxa that infected co-occurring native snails. Taken together, our results suggest that fewer trematode infections and fewer trematode taxa in P. antipodarum could contribute to the success of this invasive snail. However, despite the large geographic scale of our survey (32 rivers sampled) and the large number of sites that we visited (n = 82), few sites had either enough snails or trematode parasites in native snails to be informative. Thus, our conclusions are based on a small number of sites (n = 10).



Similar content being viewed by others
References
Adema CM, Loker ES (2015) Digenean-gastropod host associations inform on aspects of specific immunity in snails. Dev Comp Immunol 48:275–283
Adema CM, Lan CM, Hanelt B, Seville RS (2009) Digenean trematode infections of native freshwater snails and invasive Potamopyrgus antipodarum in the Grand Teton National Park/John D. Rockefeller Memorial Parkway area. J Parasitol 95:224–227
Alonso A, Castro-Diez P (2008) What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614:107–116
Alonso A, Castro-Diez P (2012) The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): state of the art of a worldwide invasion. Aquat Sci 74:375–383
Baudoin M (1975) Host castration as a parasitic strategy. Evolution 29:335–352
Benson AJ, Kipp RM, Larson J, Fusaro A (2017) Potamopyrgus antipodarum (J. E. Gray, 1853): U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL. https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=1008. Accessed 1 July 2017
Blakeslee AMH, Altman I, Miller AW, Byers JE, Hamer CE, Ruiz GM (2012) Parasites and invasions: a biogeographic examination of parasites and hosts in native and introduced ranges. J Biogeogr 39:609–622
Briers RA (2003) Range limits and parasite prevalence in a freshwater snail. Proc R Soc Lond B 270:S178–S180
Brown KM (1991) Mollusca: Gastropoda. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates. Academic Press, San Diego, pp 285–324
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on it own terms: Margolis et al. revisited. J Parasitol 83:575–583
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Davis MA (2009) Invasion biology. Oxford University Press, Oxford
Fredensborg BL, Mouritsen KN, Poulin R (2005) Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantas subcarinatas. Mar Ecol Prog Ser 290:109–117
Gendron AD, Marcogliese DJ, Thomas M (2012) Invasive species are less parasitized than native competitors, but for how long? The case of the round goby in the Great Lakes-St. Lawrence Basin. Biol Invasions 14:367–384
Genner MJ, Michel E, Todd JA (2008) Resistance of an invasive gastropod to an indigenous trematode parasite in Lake Malawi. Biol Invasions 10:41–49
Gerard C, Le Lannic J (2003) Establishment of a new host-parasite association between the introduced invasive species Potamopyrgus antipodarum (Smith) (Gastropoda) and Sanguinicola sp. Plehn (Trematoda) in Europe. J Zool 261:213–216
Gerard C, Blanc A, Costil K (2003) Potamopyrgus antipodarum (Mollusca: Hydrobiidae) in continental aquatic gastropod communities: impact of salinity and trematode parasitism. Hydrobiologia 493:167–172
Gerard C, Miura O, Lorda J, Cribb TH, Nolan MJ, Hechinger RF (2017) A native-range source for a persistent trematode parasite of the exotic New Zealand mudsnail (Potamopyrgus antipodarum) in France. Hydrobiologia 785:115–126
Goedknegt MA, Havermans J, Waser AM, Luttikhuizen PC, Velilla E, Camphuysen K, van der Meer J, Thieltges DW (2017) Cross-species comparison of parasite richness, prevalence, and intensity in a native compared to two invasive brachyuran crabs. Aquat Invasions 12:112–201
Grabner DS, Weigand AM, Leese F, Winking C, Hering D, Tollrian R, Sures B (2015) Invaders, natives and their enemies: distribution patterns of amphipods and their microsporidian parasites in the Ruhr Metropolis, Germany. Parasites Vectors 8:419. https://doi.org/10.1186/s13071-015-1036-6
Granovitch AI, Maximovich AN (2013) Long-term population dynamics of Littorina obtusata: the spatial structure and impacts of trematodes. Hydrobiologia 706:91–101
Hall RO Jr, Tank JL, Dybdahl MF (2003) Exotic snails dominate nitrogen and carbon cycling in a highly productive stream. Front Ecol Environ 1:407–411
Hall RO Jr, Dybdahl MF, VanderLoop MC (2006) Extremely high secondary production of introduced snails in rivers. Ecol Appl 16:1121–1131
Harried B, Fischer K, Perez KE, Sandland GJ (2015) Assessing infection patterns in Chinese mystery snails from Wisconsin, USA using field and laboratory approaches. Aquat Invasions 10:169–175
Hechinger RF (2012) Faunal survey and identification key for the trematodes (Platyhelminthes: Digenea) infecting Potamopyrgus antipodarum (Gastropoda: Hydrobiidae) as first intermediate host. Zootaxa 3418:1–27
Kerans BL, Dybdahl MF, Gangloff MM, Jannot JE (2005) Potamopyrgus antipodarum: distribution, density, and effects on native macroinvertebrate assemblages in the Greater Yellowstone ecosystem. J N Am Benthol Soc 24:123–138
King KC, Delph LF, Jokela J, Lively CM (2011) Coevolutionary hotspots and coldspots for host sex and parasite local adaptation in a snail-trematode interaction. Oikos 120:1335–1340
Krist AC (2000) Effect of digenean parasite Proterometra macrostoma on host morphology in the freshwater snail Elimia livescens. J Parasitol 86:262–267
Kroft KL, Blakeslee AMH (2016) Comparison of parasite diversity in native panopeid mud crabs and the invasive Asian shore crab in estuaries of northeast North America. Aquat Invasions 11:287–301
Lively CM (1987) Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328:519–521
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Wiley, Oxford
McKenzie VJ, Hall WE, Guralnick RP (2013) New Zealand mudsnails (Potamopyrgus antipodarum) in Boulder Creek, Colorado: environmental factors associated with fecundity of a parthenogenic invader. Can J Zool 91:30–36
Mitta G, Adema CM, Gourbal B, Loker ES, Theron A (2012) Compatibility polymorphism in snail/schistosome interactions: from field to theory to molecular mechanisms. Dev Comp Immunol 37:1–8
Phillips NG, Lambert DM (1989) Genetics of Potamopyrgus antipodarum (Gastropoda: Prosobranchia): evidence for reproductive modes. NZ J Zool 16:435–445
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trend Ecol Evol 19:385–390
Prior KM, Powell THQ, Joseph AL, Hellmann JJ (2015) Insights from community ecology into the role of enemy release in causing invasion success: the importance of native enemy effects. Biol Invasions 17:1283–1297
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of nonnative species. Ecol Monogr 83:263–282
Riley LA, Dybdahl MF, Hall RO Jr (2008) Invasive species impact: asymmetric interactions between invasive and endemic freshwater snails. J N Am Benthol Soc 27:509–520
Roche DG, Leung B, Mendoza Franco EF, Torchin ME (2010) Higher parasite richness, abundance and impact in native versus introduced cichlid fishes. Int J Parasitol 40:1525–1530
Roy HE, Lawson-Handley LJ (2012) Networking: a community approach to invaders and their parasites. Funct Ecol 26:1238–1248
Schell SC (1970) How to know the trematodes. WMC Brown Company Publishers, Dubuque
Strayer DL (2010) Alien species in fresh water: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174
Torchin ME, Mitchell CE (2004) Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ 2:183–190
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630
Vila M, Maron JL, Marco L (2005) Evidence for the enemy release hypothesis in Hypericum perforatum. Oecologia 142:474–479
Winterbourn MJ (1973) A guide to the freshwater mollusca of New Zealand. Tuatara 20:141–159
Winterbourn MJ (1974) Larval trematode parasitizing the New Zealand species of Potamopyrgus (Gastropoda: Hydrobiidae). Mauri Ora 2:17–30
Zbikowski J, Zbikoska E (2009) Invaders of an invader: trematodes in Potamopyrgus antipodarum in Poland. J Invertebr Pathol 101:67–70
Acknowledgements
We thank Daniel Greenwood, Kara Wise, and Megan Bochanski for help in the field collection and in the laboratory. We are also grateful to Robert Hall, Edward Levri, Lusha Tronstand, Tim Collier, and Annika Walters and two anonymous reviewers for comments that have greatly improved this manuscript. Special thanks to the University of Wyoming-National Park Service Research Station for lodging during the 2014 and 2015 field seasons. We are also grateful for funding from a Berry Graduate Research Grant and the University of Wyoming Faculty Grant-in-Aid.
Funding
This study was funded by a University of Wyoming Berry Graduate Research Grant and by a University of Wyoming Faculty Grant-in-Aid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, M.D., Krist, A.C. Trematode prevalence and an invasive freshwater snail: fewer infections and parasites likely contribute to the success of an invasive snail. Biol Invasions 22, 1279–1287 (2020). https://doi.org/10.1007/s10530-019-02179-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02179-3