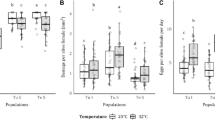
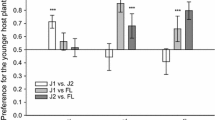
Abstract
The ability to tolerate novel herbivores is widely considered to influence plant invasion success. For clonal plants that have reduced capacity to evolve in response to novel herbivores, legacy effects of herbivory on parental plants might be translated to offspring ramets, resulting in pre-adaptation to tolerate herbivory for new vegetative growth. Using the invasive clonal plant Alternanthera philoxeroides, we first exposed plants to herbivory by Planococcus minor, a widespread and generalist piercing-sucking insect. Herbivory decreased above- and below-ground plant biomass by approximately 50% with a concomitant 134% increase in root N concentration but no changes in concentrations of soluble sugars, starch or non-structural carbohydrates related to herbivory tolerance. Offspring ramets were then exposed to herbivory by three different herbivore species: (1) P. minor, (2) the specialist leaf-beetle Agasicles hygrophila, and (3) the stenophagous tortoise-beetle Cassida piperata. There was no evidence of interactive effects between herbivory on parental plants and herbivory on offspring plants on growth, biomass allocation patterns, or physiological responses, suggesting that pre-adaptation to herbivory did not occur in A. philoxeroides with these herbivores. There were, however, species-specific herbivore tolerance responses. In the offspring generation, herbivory by A. hygrophila strongly suppressed growth and biomass allocation, but patterns were generally weaker for other herbivores. Tolerance effects could be explained by stimulatory effects of grazing by C. piperata and P. minor on taproot biomass along with idiosyncratic increases of starch and non-structural carbohydrate concentration in some storage organs. Our results highlight the importance of A. hygrophila in controlling the aboveground spread of A. philoxeroides. However, herbivory by other species was largely tolerated and accompanied by increased allocation to underground storage organs and altered physiological reserves, both of which could allow this invasive plant to tolerate herbivory and successfully invade new areas in the face of new herbivore pressure.




Similar content being viewed by others
References
Agrawal AA (2000) Specificity of induced resistance in wild radish: causes and consequences for two specialist and two generalist caterpillars. Oikos 89:493–500
Agrawal AA (2002) Herbivory and maternal effects: mechanisms and consequences of transgenerational induced plant resistance. Ecology 83:3408–3415
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715
Agrawal AA, Weber MG (2015) On the study of plant defence and herbivory using comparative approaches: how important are secondary plant compounds. Ecol Lett 18:985–991
Ali JG, Agrawal AA (2015) Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct Ecol 28:1404–1412
Ashton IW, Lerdau MT (2008) Tolerance to herbivory, and not resistance, may explain differential success of invasive, naturalized, and native North American temperate vines. Divers Distrib 14:169–178
Babst BA, Ferrieri RA, Thorpe MR, Orians CM (2010) Lymantria dispar herbivory induces rapid changes in carbon transport and partitioning in Populus nigra. Entomologia Exp Appl 128:117–125
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitations in plants-an economic analogy. Annu Rev Ecol Evol S 16:363–392
Briske DD, Richards JH (1994) Physiological responses of individual plants to grazing: current status and ecological significance. In: Vavra M, Laycock WA, Pieper RD (eds) Ecological implications of herbivory in the west. Society for Range Management, Denver, pp 147–176
Colautti RI, Ricciardi A, Grigorovich IA, Macisaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733
Cox JM (1989) The mealybug genus Planococcus (Homoptera: Pseudococcidae). B Brit Mus Entomol 58:1–78
Dai H, Lu XM, Zhang J, Ding JQ (2014) Responses of a native beetle to novel exotic plant species with varying invasion history. Ecol Entomol 39:118–124
Dam NMV, Baldwin IT (2001) Competition mediates costs of jasmonate-induced defences, nitrogen acquisition and transgenerational plasticity in Nicotiana attenuata. Funct Ecol 15:406–415
D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as a global environment change. Am Sci 84:218–228
Dong BC, Fu T, Luo FL, Yu FH (2017) Herbivory-induced maternal effects on growth and defense traits in the clonal species Alternanthera philoxeroides. Sci Total Environ 605–606:114–123
Fan SF, Yu HH, Dong XR, Wang LG, Chen XW, Yu D, Liu CH (2016) Invasive plant Alternanthera philoxeroides suffers more severe herbivory pressure than native competitors in recipient communities. Sci Rep 6:36542
Francis AW, Kairo MT, Roda AL, Liburd OE, Polar P (2012) The passionvine mealybug, Planococcus minor (Maskell)(Hemiptera: Pseudococcidae), and its natural enemies in the cocoa agroecosystem in Trinidad. Biol Control 60:290–296
Galloway LF (2005) Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol 166:93–100
Gómez S, Steinbrenner AD, Osorio S, Schueller M, Ferrieri RA, Fernie AR, Orians CM (2012) From shoots to roots: transport and metabolic changes in tomato after simulated feeding by a specialist lepidopteran. Entomol Exp Appl 44:101–111
González APR, Dumalasová V, Rosenthal J, Skuhrovec J, Latzel V (2016) The role of transgenerational effects in adaptation of clonal offspring of white clover (Trifolium repens) to drought and herbivory. Evol Ecol 31:345–361
Herman JJ, Sultan SE (2011) Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front Plant Sci 2:102
Holeski LM, Jander G, Agrawal AA (2012) Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol Evol 27:618–626
Holm LG, Doll J, Holm E, Pancho JV, Herberger JP (eds) (1997) World weeds: natural histories and distribution. Wiley, New York, pp 37–44
Huang W, Siemann E, Wheeler GS, Zuo JW, Carrillo J, Ding JQ (2010) Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. J Ecol 98:1157–1167
Huang W, He MY, Lu XM, Ding JQ (2016) Differences in interactions of aboveground and belowground herbivores on the invasive plant Alternanthera philoxeroides and native host A. sessilis. Biol Invasions 18:3437–3447
Jia X, Pan XY, Li B, Chen JK, Yang XZ (2009) Allometric growth, disturbance regime, and dilemmas of controlling invasive plants: a model analysis. Biol Invasions 11:743–752
Joshi J, Vrieling K (2005) The enemy release and EICA hypothesis revisited: incorporating the fundamental difference between specialist and generalist herbivores. Ecol Lett 8:704–714
Julien MH, Skarratt B, Maywald GF (1995) Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J Aquat Plant Manag 33:55–60
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Lapointe L, Bussières J, Crête M, Ouellet JP (2010) Impact of growth form and carbohydrate reserves on tolerance to simulated deer herbivory and subsequent recovery in Liliaceae. Am J Bot 97:913–924
Latzel V, Klimešová J (2009) Fitness of resprouters versus seeders in relation to nutrient availability in two Plantago species. Acta Oecol 35:541–547
Latzel V, Janeček Š, Doležal J, Klimešová J, Bossdorf O (2014) Adaptive transgenerational plasticity in the perennial Plantago lanceolata. Oikos 123:41–46
Lu XM, Ding JQ (2012) History of exposure to herbivores increases the compensatory ability of an invasive plant. Biol Invasions 14:649–658
Lu XM, Siemann E, Shao X, Huang W, Ding JQ (2013) Climate warming affects biological invasions by shifting interactions of plants and herbivores. Global Change Biol 19:2339–2347
Lu XM, Shao X, Ding JQ (2014) No impact of a native beetle on exotic plant performance and competitive ability due to plant compensation. Plant Ecol 215:275–284
Luo FL, Chen Y, Huang L, Wang A, Zhang MX, Yu FH (2014) Shifting effects of physiological integration on performance of a clonal plant during submergence and de-submergence. Ann Bot 113:1265–1274
Machado RAR, Zhou W, Ferrieri AP, Arce CCM, Baldwin IT, Xu S, Erb M (2017) Species-specific regulation of herbivory-induced defoliation tolerance is associated with jasmonate inducibility. Ecol Evol 7:3703–3712
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Mccarthy MC, Enquist BJ (2007) Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol 21:713–720
Millard P, Hester A, Wendler R, Baillie G (2010) Interspecific defoliation responses of trees depend on sites of winter nitrogen storage. Funct Ecol 15:535–543
Morrison WE, Hay ME (2011) Herbivore preference for native vs. exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily Naïve. PLoS ONE 6:e17227
Mousseau TA, Fox CW (1998) The adaptive significance of maternal effects. Trends Ecol Evol 13:403–407
Mueller RC, Wade BD, Gehring CA, Whitham TG (2005) Chronic herbivory negatively impacts cone and seed production, seed quality and seedling growth of susceptible pinyon pines. Oecologia 143:558–565
Nagasawa A, Matsuda K (2015) Factors determining the host range of two tortoise beetles, Cassida nebulosa L. and C. piperata Hope (Coleoptera: Chrysomelidae) in Japan. Open Entomol J 9:1–6
Newingham BA, Callaway RM, Bassirirad H (2007) Allocating nitrogen away from a herbivore: a novel compensatory response to root herbivory. Oecologia 153:913–920
Obeso JR (1993) Does defoliation affect reproductive output in herbaceous perennials and woody plants in different ways? Funct Ecol 7:150–155
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65
Polley HW, Detling JK (1988) Herbivory tolerance of Agropyron smithii populations with different grazing histories. Oecologia 77:261–267
Roda A, Francis AW, Kairo MTK, Culik M, Peña JE (2013) Planococcus minor (Hemiptera: Pseudococcidae): bioecology, survey and mitigation strategies. In: Peña JE (ed) Potential invasive pests of agricultural crops. CAB International, Wallingford, pp 288–300
Sainty G, McCorkelle G, Julien M (1998) Control and spread of alligator weed Alternanthera philoxeroides (Mart.) Griseb., in Australia: lessons for other regions. Wetl Ecol Manag 5:195–201
Schooler S, Baron Z, Julien M (2006) Effect of simulated and actual herbivory on alligator weed, Alternanthera philoxeroides, growth and reproduction. Biol Control 36:74–79
Schoonhoven LM, Loon JJAV, Dicke M (2005) Insect-plant biology, 2nd. Oxford University Press, Oxford
Schwachtje J, Minchin PE, Jahnke S, van Dongen JT, Schittko U, Baldwin IT (2006) SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. P Natl Acad Sci USA 103:12935–12940
Spencer NR, Coulson JR (1976) The biological control of alligatorweed, Alternanthera philoxeroides, in the United States of America. Aquat Bot 2:177–190
Steets J, Ashman TL (2010) Maternal effects of herbivory in Impatiens capensis. Int J Plant Sci 171:509–518
Thornton B, Millard P (1997) Increased defoliation frequency depletes remobilization of nitrogen for leaf growth in grasses. Ann Bot 80:89–95
Thornton B, Millard P, Duff EI, Buckland ST (1993) The relative contribution of remobilization and root uptake in supplying nitrogen after defoliation for regrowth of laminae in four grass species. New Phytol 124:689–694
Venette RC, Davis EE (2004) Mini risk assessment: passionvine mealybug, Planococcus minor (Maskell) (Pseudococcidae: Hemiptera). Department of Entomology, University of Minnesota, St. Paul, pp 1–30
Wang N, Yu FH, Li PX, He WM, Liu J, Yu GL, Song YB, Dong M (2009) Clonal integration supports the expansion from terrestrial to aquatic environments of the amphibious stoloniferous herb Alternanthera philoxeroides. Plant Biol 11:483–489
Wang P, Li H, Pang XY, Wang A, Dong BC, Lei JP, Yu FH, Li MH (2017) Clonal integration increases tolerance of a phalanx clonal plant to defoliation. Sci Total Environ 593–594:236–241
Wilson JRU, Yeates A, Schooler S, Julien MH (2007) Rapid response to shoot removal by the invasive wetland plant, alligator weed (Alternanthera philoxeroides). Environ Exp Bot 60:20–25
Xu CY, Zhang WJ, Fu CZ, Lu BR (2003) Genetic diversity of alligator weed in China by RAPD analysis. Biodivers Conserv 12:637–645
Ye WH, Li J, Cao HL, Cao HL, Ge XJ (2003) Genetic uniformity of Alternanthera philoxeroides in South China. Weed Res 43:297–302
Yu FH, Wang N, Alpert P, He WM, Dong M (2009) Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. Am J Bot 96:1983–1989
Acknowledgements
We thank Jia-Hao Wang and Ting Fu for assistance with the management of the experiment and plant harvest, Dr. Bo-Yi Chen, Dr. Jian-Yu Li, and Dr. Yong-Jian Wang for assistance with insect collection, and Qiao-Qi Sun, the associate editor and two anonymous reviewers for their valuable comments. This work was supported by National Key Research and Development Program of China (2016YFC1202102, 2016YFC1201101), National Natural Science Foundation of China (31500331), and Fundamental Research Funds for the Central Universities (2015ZCQ-BH-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, BC., Wang, MZ., Liu, RH. et al. Direct and legacy effects of herbivory on growth and physiology of a clonal plant. Biol Invasions 20, 3631–3645 (2018). https://doi.org/10.1007/s10530-018-1801-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-018-1801-5