Abstract
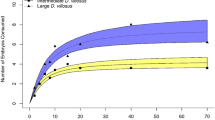
Possession of unique defensive toxins by nonindigenous species may increase the likelihood of creating evolutionary traps for native predators. We tested the hypothesis that nonindigenous, toxic Cuban Treefrogs (Osteopilus septentrionalis) have created an evolutionary trap for native, generalist snakes. Additionally, we explored the possibility that populations of snakes that co-occur with Cuban Treefrogs have responded in ways that allow them to escape potential trap dynamics. To evaluate a potential fitness cost of consuming Cuban Treefrogs, we monitored growth of 61 wild-caught Common Gartersnakes (Thamnophis sirtalis) fed exclusive diets of either Cuban Treefrogs, native Green Treefrogs (Hyla cinerea), or native Golden Shiners (Notemigonus crysoleucas). Snakes in the Cuban Treefrog diet treatment gained less than half the mass of those consuming native prey, and Cuban Treefrogs were significantly less digestible than native prey. There was no difference in the response of gartersnakes to prey scent cues of Cuban Treefrogs and Green Treefrogs. Our results indicate that Cuban Treefrogs likely represent an evolutionary trap for snakes because consumption of these frogs carries fitness costs, yet snakes fail to recognize this prey as being costly. We found no difference in growth or response to prey cues between snakes from invaded and non-invaded regions, suggesting snakes have not responded to escape trap dynamics. Interactions of native snakes and Cuban Treefrogs support the idea that introduced species with novel toxins may increase the likelihood of evolutionary trap formation.




Similar content being viewed by others
References
Antczak M, Hromada M, Tryjanowski P (2005) Frogs and toads in the food of the Great Grey Shrike (lanius excubitor): larders and skinning as two ways to consume dangerous prey. Anim Biol Leiden 55:227–233
Arnold SJ (1978) Some effects of early experience on feeding responses in the Common Garter Snake, Thamnophis sirtalis. Anim Behav 26:455–462
Arnold SJ (1981) Behavioral variation in natural populations. I. Phenotypic, genetic and environmental correlations between chemoreceptive responses to prey in the garter snake, Thamnophis elegans. Evolution 35:489–509
Beckmann C, Shine R (2011) Toad’s tongue for breakfast: exploitation of a novel prey type, the invasive Cane Toad, by scavenging raptors in tropical Australia. Biol Invasions 13:1447–1455
Betz FS, Hammond BG, Fuchs RL (2000) Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul Toxicol Pharm 32:156–173
Boyd CE, Goodyear CP (1971) The protein content of some common reptiles and amphibians. Herpetologica 27:317–320
Brodie ED III, Brodie ED (1990) Tetrodotoxin resistance in garter snakes: an evolutionary response of predators to dangerous prey. Evolution 44:651–659
Brodie ED III, Brodie ED (1999) Predator-prey arms races: asymmetrical selection on predators and prey may be reduced when prey are dangerous. Bioscience 49:557–568
Bronikowski AM (2000) Experimental evidence for the adaptive evolution of growth rate in the garter snake Thamnophis elegans. Evolution 54:1760–1767
Burghardt GM (1967) Chemical-cue preferences of inexperienced snakes: comparative aspects. Science 157:718–721
Burghardt GM (1969) Comparative prey-attack studies in newborn snakes of the genus Thamnophis. Behaviour 33:77–113
Burghardt GM (1970) Chemical perception in reptiles. In: Johnston J, Moulton D, Turk A (eds) Advances in chemoreception, vol I. Communication by chemical signals. Appleton Century Crofts, New York, pp 241–308
Burghardt GM, Wilcoxon HC, Czaplicki JA (1973) Conditioning in garter snakes: aversion to palatable prey induced by delayed illness. Anim Learn Behav 1:317–320
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Cappuccino N, Arnason JT (2006) Novel chemistry of invasive exotic plants. Biol Lett 2:189–193
Cappuccino N, Carpenter D (2005) Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol Lett 1:435–438
Carpenter D, Cappuccino N (2005) Herbivory, time since introduction and the invasiveness of exotic plants. J Ecol 93:315–321
Cooper WE Jr (1998) Evaluation of swab and related tests as a bioassay for assessing responses by squamate reptiles to chemical stimuli. J Chem Ecol 24:841–866
Cooper WE Jr, Burghardt GM (1990) A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J Chem Ecol 16:45–65
Cooper WE Jr, Burghardt GM, Brown WS (2000) Behavioural responses by hatchling racers (Coluber constrictor) from two geographically distinct populations to chemical stimuli from potential prey and predators. Amphib Reptil 21:103–115
Cooper WE Jr, Pérez-Mellado V, Vitt LJ, Budzynski B (2003) Cologne as a pungency control in tests of chemical discrimination: effects of concentration, brand, and simultaneous and sequential presentation. J Ethol 21:101–106
Cornell HV, Hawkins BA (2003) Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am Nat 161:507–522
de Queiroz A, Lawson R, Lemos-Espinalet JA (2002) Phylogenetic relationships of North American garter snakes (Thamnophis) based on four mitochondrial genes: how much DNA sequence is enough? Mol Phylogenet Evol 22:315–329
Delfino G, Brizzi R, Nosi D, Terreni A (2002) Serous cutaneous glands in new world hylid frogs: an ultrastructural study on skin poisons confirms phylogenetic relationships between Osteopilus septentrionalis and phrynohyas venulosa. J Morphol 253:176–186
Ernst CH, Ernst EM (2003) Snakes of the United States and Canada. Smithsonian Books, Washington
Faivovich J, Haddad CF, Garcia PC, Frost DR, Campbell JA, Wheeler WC (2005) Systematic review of the frog family Hylidae, with special reference to hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist 294:1–240
Fitch H (1965) An ecological study of the garter snake, Thamnophis sirtalis. Univ Kansas Mus Nat Hist 15:493–564
Frost DR et al (2006) The amphibian tree of life. Bull Am Mus Nat Hist 297:1–291
Frutos R, Rang C, Royer M (1999) Managing insect resistance to plants producing Bacillus thuringiensis toxins. Crit Rev Biotechnol 19:227–276
Gibbons JW et al (2006) Remarkable amphibian biomass and abundance in an isolated wetland: implications for wetland conservation. Conserv Biol 20:1457–1465
Greene HW (1997) Snakes: the evolution of mystery in nature. University of California Press, Berkeley
Gregory PT (1977) Life-history parameters of the Red-Sided Garter Snake (Thamnophis sirtalis parietalis) in an extreme environment, the interlake region of Manitoba. National Museums of Canada
Halpern M (1992) Nasal chemical senses in reptiles: structure and function. In: Gans C, Crews D (eds) Biology of the reptilia, vol 18. Physiology E, hormones, brain, and behavior. University of Chicago Press, Chicago, pp 423–523
Halpern M, Frumin N (1979) Roles of the vomeronasal and olfactory systems in prey attack and feeding in adult garter snakes. Physiol Behav 22:1183–1189
Hanifin CT, Brodie ED Jr, Brodie ED III (2008) Phenotypic mismatches reveal escape from arms-race coevolution. PLoS Biol 6:e60
Huang F, Buschman LL, Higgins RA, McGaughey WH (1999) Inheritance of resistance to Bacillus thuringiensis toxin (Dipel ES) in the European Corn Borer. Science 284:965–967
Iverson JB (1987) Patterns of relative fecundity in snakes. Florida Sci 50:223–233
Jogesh T, Carpenter D, Cappuccino N (2008) Herbivory on invasive exotic plants and their non-invasive relatives. Biol Invasions 10:797–804
Kiesecker JM, Blaustein AR (1997) Population differences in responses of Red-Legged Frogs (Rana aurora) to introduced bullfrogs. Ecology 78:1752–1760
Koziel MG et al (1993) Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringiensis. Nat Biotechnol 11:194–200
Leary CJ, Razafindratsita VR (1998) Attempted predation on a hylid frog, Phrynohyas venulosa, by an indigo snake, Drymarchon corais, and the response of conspecific frogs to distress calls. Amphib Reptil 18:442–446
Licht LE, Low B (1968) Cardiac response of snakes after ingestion of toad parotoid venom. Copeia 1968:547–551
Lizana M, Mellado VP (1990) Predation by the otter (Lutra lutra) of the toad of the Sierra de Gredos (Bufo bufo gredosicola). Donana Acta Vertebr 17:109–112
Love WB (1995) Osteopilus septentrionalis (Cuban Treefrog). Predation. Herpetol Rev 26:201–202
Manzanilla J, La Marca E, Villareal O, Sanchez D (1998) Phrynohyas venulosa (Veined treefrog, “Rana lechosa”). Antipredator device. Herpetol Rev 29:39–40
Meshaka WE (2001) The Cuban treefrog in Florida: life history of a successful colonizing species. University Press of Florida, Gainesville
O’Callaghan M, Glare TR, Burgess EPJ, Malone LA (2005) Effects of plants genetically modified for insect resistance on nontarget organisms. Annu Rev Entomol 50:271–292
Phillips BL, Shine R (2004) Adapting to an invasive species: Toxic Cane Toads induce morphological change in Australian snakes. Proc Natl Acad Sci USA 101:17150–17155
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: Cane Toads and Black Snakes in Australia. Proc R Soc B Biol Sci 273:1545–1550
Pough FH (1980) The advantages of ectothermy for tetrapods. Am Nat 115:92–112
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Available online at: https://www.R-project.org/
Rice KG, Waddle JH, Miller MW, Crockett ME, Mazzotti FJ, Percival HF (2011) Recovery of native treefrogs after removal of nonindigenous Cuban Treefrogs, Osteopilus septentrionalis. Herpetologica 67:105–117
Robbins TR, Langkilde T (2012) The consequences of lifetime and evolutionary exposure to toxic prey: changes in avoidance behavior through ontogeny. J Evolut Biol 25:1937–1946
Robertson BA, Rehage JS, Sih A (2013) Ecological novelty and the emergence of evolutionary traps. Trends Ecol Evol 28:552–560
Rossman DA, Ford NB, Seigel RA (1996) The garter snakes: evolution and ecology. University of Oklahoma Press, Norman
Rowe AH, Rowe MP (2008) Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon 52:597–605
Schaffner U, Ridenour WM, Wolf VC, Bassett T, Müller C, Müller-Schärer H, Sutherland S, Lortie CJ, Callaway RM (2011) Plant invasions, generalist herbivores, and novel defense weapons. Ecology 92:829–835
Schlaepfer MA, Runge MC, Sherman PW (2002) Ecological and evolutionary traps. Trends Ecol Evol 17:474–480
Schlaepfer MA, Sherman PW, Blossey B, Runge MC (2005) Introduced species as evolutionary traps: introduced species as evolutionary traps. Ecol Lett 8:241–246
Seigel RA, Fitch HS (1985) Annual variation in reproduction in snakes in a fluctuating environment. J Anim Ecol 54:497–505
Seigel RA, Ford NB (1987) Reproductive ecology. In: Seigel JT, Collins JT, Novak SS (eds) Snakes: ecology and evolutionary biology. Macmillan Publishing Company, New York, pp 210–252
Shelton AM, Zhao JZ, Roush RT (2002) Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu Rev Entomol 47:845–881
Shine R (2010) The ecological impact of invasive Cane Toads (bufo marinus) in Australia. Q Rev Biol 85:253–291
Stewart GR (1968) Some observations on the natural history of two Oregon garter snakes (genus Thamnophis). J Herpetol 2:71–86
Strauss SY, Lau JA, Carroll SP (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol Lett 9:357–374
Tabashnik BE (1994) Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol 39:47–79
Thompson JN (1997) Evaluating the dynamics of coevolution among geographically structured populations. Ecology 78:1619–1623
Thompson JN (1999) Specific hypotheses on the geographic mosaic of coevolution. Am Nat 153:S1–S14
Waddle JH, Dorazio RM, Walls SC, Rice KG, Beauchamp J, Schuman MJ, Mazzotti FJ (2010) A new parameterization for estimating co-occurrence of interacting species. Ecol Appl 20:1467–1475
Waters RM, Burghardt GM (2013) Prey availability influences the ontogeny and timing of chemoreception-based prey shifting in the Striped Crayfish Snake, Regina alleni. J Comp Psychol 127:49–55
Weldon AJ, Haddad NM (2005) The effects of patch shape on Indigo Buntings: evidence for an ecological trap. Ecology 86:1422–1431
Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E (1998) Quantifying threats to imperiled species in the United States. Bioscience 48:607–615
Yu CG, Mullins MA, Warren GW, Koziel MG, Estruch JJ (1997) The Bacillus thuringiensis vegetative insecticidal protein Vip3A lyses midgut epithelium cells of susceptible insects. Appl Environ Microb 63:532–536
Acknowledgements
We thank B. Folt, J. Friars, A. Jenkins, S. Johnson, M. Miller, J. Stiles, and S. Stiles for assistance in the field and collection of study animals and R. Pudner for assistance with figures. We thank M. Mendonca and R. Reed for helpful comments on study design and D. Steen for constructive comments on this manuscript. Partial funding was provided by an Auburn University Natural History Collections Acquisition Grant to SG. All research was conducted in accordance with IACUC protocols (2013-2386, 2015-2659), Alabama Department of Conservation and Natural Resources Scientific Collecting Permit (8290), and Everglades National Park Scientific Research Permits (EVER-2012-SCI-0023, EVER-2013-SCI-0036, EVER-2014-SCI-0058).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goetz, S.M., Guyer, C., Boback, S.M. et al. Toxic, invasive treefrog creates evolutionary trap for native gartersnakes. Biol Invasions 20, 519–531 (2018). https://doi.org/10.1007/s10530-017-1554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1554-6