Abstract
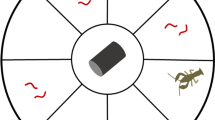
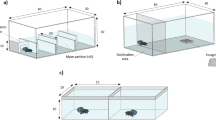
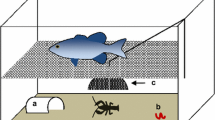
Invasive species present a global threat to natural ecosystems and native biodiversity. Previous studies have shown that invasive range expansion is often related to the invader’s life histories and dispersal behavior. Among behavioral traits, boldness is a key trait that may aid species in performing well in novel environments. Thus, along a species’ invaded range, individuals from the invasion front should be bolder, better dispersers, and have life histories that maximize population growth relative to established populations. We tested these hypotheses with the invasion of the African jewelfish Hemichromis letourneuxi in Everglades National Park (ENP). Jewelfish entered ENP in 2000, and since then they have expanded their range rapidly but traceably. Our study examined variation in reproductive investment, body condition, gut fullness, boldness, and dispersal behavior across six wild-caught populations of African jewelfish. Boldness and dispersal were tested using an emergence-activity test and an emergence-dispersal test in large, outdoor experimental setups. We dissected fish from the six populations to assess life histories. Populations from the invasion front (western ENP) had higher reproductive investment, higher gut fullness, and better body condition, but they were not relatively bolder nor better dispersers than inner populations (eastern ENP). As the invasion progressed, lower intraspecific density at the invasion front may have relaxed competition and allowed for higher fitness and reproductive investment. Understanding underlying behavioral and life-history mechanisms of an invasion is key for the development of management strategies that aim to contain current invaders and prevent the spread of future ones.






Similar content being viewed by others
References
Alford RA, Brown GP, Schwarzkopf L, Phillips BL, Shine R (2009) Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildl Res 36:23–28
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Bell AM (2005) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J Evol Biol 18:464–473
Bell AM, Stamps JA (2004) Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim Behav 68:1339–1348
Biro PA, Dingemanse NJ (2009) Sampling bias resulting from animal personality. Trends Ecol Evol 24:66–67
Biro PA, Beckmann C, Stamps JA (2010) Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc B 277:71–77
Bland JM, Altman DG (1996) Transformations, means, and confidence intervals. Br Med J 312:770
Bohn T, Sandlund OT, Amundsen P, Primiceiro R (2004) Rapidly changing life history during invasion. Oikos 106:138–150
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Brooks WR, Jordan RC (2010) Enhanced interspecific territoriality and the invasion success of the spotted tilapia (Tilapia mariae) in South Florida. Biol Invasions 12:865–874
Brown C, Jones F, Braithwaite V (2005) In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim Behav 70:1003–1009
Brown GP, Phillips BL, Webb JK, Shine R (2006) Toad on the road: use of roads as dispersal corridors by cane toads (Bufo marinus) at an invasion front in tropical Australia. Biol Conserv 133:88–94
Burton OJ, Phillips BL, Travis JMJ (2010) Trade-offs and the evolution of life-histories during range expansion. Ecol Lett 13:1210–1220
Case TJ, Taper ML (2000) Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Amer Nat 155:583–605
Chick JH, Ruetz CR, Trexler J (2004) Spatial scale and abundance patterns of large fish communities in freshwater marshes of the Florida Everglades. Wetlands 24:652–664
Child T, Phillips BL, Shine R (2008) Abiotic and biotic influences on the dispersal behavior of metamorph cane toads (Bufo marinus) in tropical Australia. J Exp Zool 309A:215–224
Collins TM, Trexler JC, Nico LG, Rawlings TA (2002) Genetic diversity in a morphologically conservative invasive taxon: multiple introductions of swamp eels to the Southeastern United States. Conserv Biol 16:1024–1035
Copeland T, Murphy BR, Ney JJ (2010) The effects of feeding history and environment on condition, body composition and growth of bluegills Lepomis macrochirus. J Fish Biol 76:538–555
Cote J, Clobert J (2010) Risky dispersal: avoiding kin competition despite uncertainty. Ecology 91:1485–1493
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc B Biol Sci 277:1571–1579
Cote J, Fogarty S, Brodin T, Weinersmith K, Sih A (2011) Personality-dependent dispersal in the invasive mosquitofish: group composition matters. Proc R Soc B Biol Sci 278:1670–1678
Davis MA (2009) Invasion biology. Oxford University Press, New York
Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Duckworth RA (2008) Adaptive dispersal strategies and the dynamics of range expansion. Amer Nat 172:S2–S17
Duckworth RA, Badyaev AV (2007) Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci USA A104:15017–15022
Dunlop-Hayden K, Rehage JS (2011) Antipredator behavior and cue recognition by multiple Everglades prey to a novel cichlid predator. Behaviour 148:795–823
Fogarty S, Cote J, Sih A (2011) Social personality polymorphism and the spread of invasive species: a model. Amer Nat 177:273–287
Fox MG, Vila-Gispert A, Coop GH (2007) Life-history traits of introduced Iberian pumpkinseed Lepomis gibbosus relative to native populations. Can differences explain colonization success? J Fish Biol 71:56–69
Fraser DF, Gilliam JF, Daley MJ, Le AN, Skalski GT (2001) Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Amer Nat 158:124–135
Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU (2007) Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc R Soc B 274:333–339
Gammon DE, Maurer BA (2002) Evidence for non-uniform dispersal in the biological invasion of two naturalized North American bird species. Global Ecol Biogeogr 11:155–161
Gaston KJ (2009) Geographic range limits: achieving synthesis. Proc R Soc B 276:1395–1406
Ghalambor CK, McCay JK, Carroll SP, Reznick DN (2007) Adaptive vs. non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Gilliam JF, Fraser DF (2001) Movement in corridors: enhancement by predation threat, disturbance, and habitat structure. Ecology 82:258–273
Gilroy JJ, Sutherland WJ (2007) Beyond ecological traps: perceptual errors and undervalued resources. Trends Ecol Evol 22:351–356
Gurevitch J, Fox GA, Wardle GM, Inderjit, Taub D (2011) Emergent insights from the synthesis of conceptual frameworks for biological invasions. Ecol Lett 14:407–418
Harcourt JL, Biau S, Johnstone R, Manica A (2010) Boldness and information use in three-spined sticklebacks. Ethology 115:1–8
Hendry AP, Farrugia TJ, Kinnison MT (2008) Human influences on rates of phenotypic change in wild animal populations. Mol Ecol 17:20–29
Holt RD, Keitt TH, Lewis MA, Maurer BA, Taper ML (2005) Theoretical models of species’ borders: single species approaches. Oikos 108:18–27
Holway DA, Suarez AV (1999) Animal behavior: an essential component of invasion biology. Trends Ecol Evolut 14:328–330
Hughes CL, Dytham C, Hill JK (2007) Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol Entomol 32:437–445
Ioannou CC, Payne M, Krause J (2008) Ecological consequences of the bold–shy continuum: the effect of predator boldness on prey risk. Oecology 157:177–182
Joanna G, Dariusz P, Miroslaw P, Serhan TA, Lidia M, Magdalena L (2011) Life-history traits of Amur sleeper, Perccottus glenii, in the invaded Vistula River: early investment in reproduction but reduced growth rate. Hydrobiologia 661:197–210
Kobler A, Engelen B, Knaepkens G, Eens M (2009) Temperament in bullheads: do laboratory and field explorative behaviour variables correlate? Naturwissenschaften 96:1229–1233
Kobza RM, Trexler JC, Loftus WF, Perry SA (2004) Community structure of fishes inhabiting aquatic refuges in a threatened Karst wetland and its implications for ecosystem management. Biol Cons 116:153–165
Kolbe JJ, Glor RE, Rodriguez L, Chamizo A, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–180
Kreiner A, Van Der Lingen CD, Freon P (2001) A comparison of condition factor and gonadosomatic index of sardine Sardinops sagax stocks in the northern and southern Benguela upwelling ecosystems, 1984–1999. S Afr J Mar Sci 23:123–134
Kubisch A, Hovestadt T, Poethke H (2010) On the elasticity of range limits during periods of expansion. Ecology 91:3094–3099
Kurvers R, Eijkelenkamp B, van Oers K, van Lith B, van Wieren SE, Ydenberg RC, Prins HHT (2009) Personality differences explain leadership in barnacle geese. Anim Behav 78:447–453
Llewelyn J, Phillips BL, Alford RA, Schwarzkopf L, Shine R (2010) Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162:343–348
Lodge DM, Williams S, MacIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, Carlton JT, McMichael A (2006) Biological invasions: recommendations for US policy and management. Ecol Appl 16:2035–2054
Loiselle PV (2000) Natural history and aquarium husbandry of the savannah jewel fish, Hemichromis letourneauxi Sauvage, 1880. Cichlid News Mag 9:24–31
McCauley SJ, Brodin T, Hammond J (2010) Foraging rates of larval dragonfly colonists are positively related to habitat isolation: results from a landscape-level experiment. Am Nat 175:E66–E73
Meylan S, De Fraipont M, Aragon P, Vercken E, Clobert J (2009) Are dispersal-dependent behavioral traits produced by phenotypic plasticity? J Exp Zool A Ecol Genet Physiol 311A:377–388
Nathan R, Perry G, Cronin JT, Strand AE, Cain ML (2003) Methods for estimating long-distance dispersal. Oikos 103:261–273
Nickum JG, Bart HL, Bowser PR, Greer IE, Hubbs C, Jenkins JA, MacMillan JR, Rachlin JW, Rose JD, Sorensen PW, Tomasso JR (2004) Guidelines for the use of fishes in research. American Fisheries Society, Bethesda
Nilsson E, Persson A, Nilsson PA (2010) Interspecific competition and predation: relative effects on foragers and their densities. Behav Ecol Sociobiol 64:729–736
Obaza A, DeAngelis DL, Trexler JC (2011) Using data from an encounter sampler to model fish dispersal. J Fish Biol 78:495–513
Olden JD, Poff NL, Bestgen KR (2006) Life-history strategies predict fish invasions and extirpations in the Colorado River Basin. Ecol Monographs 76:25–40
Palmer ML, Mazzotti FJ (2004) Structure of Everglades alligator holes. Wetlands 24:115–122
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Phillips BL (2009) The evolution of growth rates on an expanding range edge. Biol Lett 5:802–804
Phillips BL, Brown GP, Webb JK, Shine R (2006) Invasion and the evolution of speed in toads. Nature 439:803
Phillips BL, Brown GP, Greenlees M, Webb JK, Shine R (2007) Rapid expansion of the cane toad (Bufo marinus) invasion front in tropical Australia. Austral Ecol 32:169–176
Phillips BL, Brown GP, Travis JMJ, Shine R (2008) Reid’s paradox revisited: the evolution of dispersal kernels during range expansion. Am Nat 172:S34–S48
Phillips BL, Brown GP, Shine R (2010a) Evolutionarily accelerated invasions: the rate of dispersal evolves upwards during the range advance of cane toads. J Evol Biol 23:2595–2601
Phillips BL, Brown GP, Shine R (2010b) Life-history evolution in range-shifting populations. Ecology 9:1617–1627
Polacik M, Janac M, Jurajda P, Adamek Z, Ondrackova M, Trichkova T, Vassilev M (2009) Invasive gobies in the Danube: invasion success facilitated by availability and selection of superior food resources. Ecol Freshw Fish 18:640–649
Raby GD, Gutowsky LFG, Fox MG (2010) Diet composition and consumption rate in round goby (Neogobius melanostomus) in its expansion phase in the Trent River, Ontario. Environ Biol Fishes 89:143–150
Rehage JS, Loftus WF (2007) Seasonal fish community variation in mangrove creeks in the southwestern Everglades: an examination of their role as dry-down refuges. Bull Mar Sci 80:625–645
Rehage JS, Sih A (2004) Dispersal behavior, boldness, and the link to invasiveness: a comparison of four Gambusia species. Biol Invasions 6:379–391
Rehage JS, Trexler JC (2006) Assessing the net effect of anthropogenic disturbance on aquatic communities in wetlands: community structure relative to distance from canals. Hydrobiologia 569:359–373
Rehage JS, Dunlop KL, Loftus WF (2009) Antipredator responses by native mosquitofish to non-native cichlids: an Examination of the Role of Prey Naivete. Ethol 115:1046–1056
Ribeiro F, Collares-Pereira MJ (2010) Life-history variability of non-native centrarchids in regulated river systems of the lower River Guadiana drainage (south-west Iberian Peninsula). J Fish Biol 76:522–537
Rivas LR (1965) Florida fresh water fishes and conservation. Q J Fla Acad Sci 28:255–258
Ruetz CR, Trexler JC, Jordan F, Loftus WF, Perry S (2005) Population dynamics of wetland fishes: spatio-temporal patterns synchronized by hydrological disturbance? J Anim Ecol 74:322–332
Rutchey K, Schall T, Sklar F (2008) Development of vegetation maps for assessing Everglades restoration progress. Wetlands 28:806–816
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sato M, Kawaguchi Y, Nakajima J, Mukai T, Shimatani Y, Onikura N (2010) A review of the research on introduced freshwater fishes: new perspectives, the need for research, and management implications. Landsc Ecol Eng 6:99–108
Schofield PJ, Loftus WF, Brown ME (2007) Hypoxia tolerance of two centrarchid sunfishes and an introduced cichlid from karstic Everglades wetlands of southern Florida, USA. J Fish Biol 71(Supplement D):87–99
Schurch R, Heg D (2010) Life history and behavioral type in the highly social cichlid Neolamprologus pulcher. Behav Ecol 21:588–598
Seppanen JT, Forsman JT, Monkkonen M, Thomson RL (2007) Social information use is a process across time, space, and ecology, reaching heterospecifics. Ecology 88:1622–1633
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Ann Rev Ecol Evol Syst 40:415–436
Shafland PL, Gestring KB, Stanford MS (2008) Florida’s exotic freshwater fishes. Fla Sci 3:220–245
Shine R, Brown GP, Phillips BL (2011) An evolutionary process that assembles phenotypes through space rather than through time. Proc Natl Acad Sci 108:5708–5711
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Stiver KA, Desjardins JK, Fitzpatrick JL, Neff B, Quinn JS, Balshine S (2007) Evidence for size and sex-specific dispersal in a cooperatively breeding cichlid fish. Mol Ecol 16:2974–2984
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Tabachnick BG, Fidell LS (1996) Using multivariate statistics. Harper Collins, New York
Travis JMJ, Dytham C (2002) Dispersal evolution during invasions. Evol Ecol Res 4:1119–1129
Tuomainen U, Candolin U (2011) Behavioural responses to human-induced environmental change. Biol Rev 86:640–657
Urban MC, Phillips BL, Skelly D, Shine R (2008) A toad more traveled: the heterogeneous invasion dynamics of cane toads in Australia. Am Nat 171:E134–E148
Williams JE (2000) The coefficient of condition of fish, chap 13. In: Schneider JC (ed) Manual of fisheries survey methods II: with periodic updates. Michigan Department of Natural Resources, Fisheries Special Report 25, Ann Arbor
Wilson ADM, Godin JGJ (2009) Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav Ecol 20:231–237
Wilson DS, Coleman K, Clark AB, Biederman L (1993) Shy bold continuum in pumpkinseed sunfish (Lepomis gibbosus) an ecological study of a psychological trait. J Comp Psych 107:250–260
Wilson ADM, Godin JGJ, Ward AJW (2010) Boldness and reproductive fitness correlates in the Eastern Mosquitofish, Gambusia holbrooki. Ethol 116:96–104
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life history trade-offs favour the evolution of animal personalities. Nature 447:581–584
Yanagisawa Y, Sato T (1990) Active browsing by mouthbrooding females of Tropheus duboisi and Tropheus moorii to feed the young and or themselves. Environ Biol Fishes 27:43–50
Acknowledgments
This research is contribution # 549 of the Southeast Environmental Research Center(SERC) and was partly funded by a SERC Everglades Fellowship, Florida International University, by funding from CERP RECOVER through the Army Corps of Engineers and the South Florida Water Management District, and was developed in collaboration with the Florida Coastal Everglades LTER program under NSF DEB-9910514. We thank P. Stoddard, J. Heinen, and J. Kline for their support and feedback on this project. We greatly appreciate the valuable assistance of N. Bernal, J. P. Perea, R. Boucek, B. Gallagher, D. Gandy, and M. Anderson in the field and the overall development of this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez, D.P., Jungman, A.A. & Rehage, J.S. Nonnative African jewelfish are more fit but not bolder at the invasion front: a trait comparison across an Everglades range expansion. Biol Invasions 14, 2159–2174 (2012). https://doi.org/10.1007/s10530-012-0221-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0221-1