Abstract
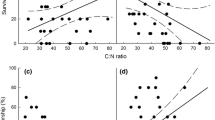
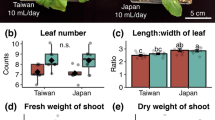
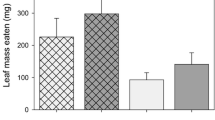
The northern tamarisk beetle (Diorhabda carinulata Desbrochers) was released in several western states as a biocontrol agent to suppress Tamarix spp. L. which has invaded riparian ecosystems; however, effects of beetle herbivory on Tamarix physiology are largely undocumented and may have ecosystem ramifications. Herbivory by this insect produces discoloration of leaves and premature leaf drop in these ecosystems, yet the cause of premature leaf drop and the effects of this leaf drop are still unknown. Insect herbivory may change leaf photosynthesis and respiration and may affect a plant’s ability to regulate water loss and increase water stress. Premature leaf drop may affect plant tissue chemistry and belowground carbon allocation. We conducted a greenhouse experiment to understand how Tamarix responds physiologically to adult beetle and larvae herbivory and to determine the proximate cause of premature leaf drop. We hypothesized that plants experiencing beetle herbivory would have greater leaf and root respiration rates, greater photosynthesis, increased water stress, inefficient leaf nitrogen retranslocation, lower root biomass and lower total non-structural carbohydrates in roots. Insect herbivory reduced photosynthesis rates, minimally affected respiration rates, but significantly increased water loss during daytime and nighttime hours and this produced increased water stress. The proximate cause for premature leaf drop appears to be desiccation. Plants exposed to herbivory were inefficient in their retranslocation of nitrogen before premature leaf drop. Root biomass showed a decreasing trend in plants subjected to herbivory. Stress induced by herbivory may render these trees less competitive in future growing seasons.









Similar content being viewed by others
References
Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143:1–7
Carruthers RI, Deloach CJ, Herr JC, Anderson GL, Knutson AE (2008) Salt cedar areawide management in the western US. In: Koul O, Cuperus GW, Elliott N (eds) Areawide pest management: theory and implementation. CABI, Cambridge, pp 270–309
Chapin FS, Kedrowski RA (1983) Seasonal-changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous Taiga trees. Ecology 64:376–391
Chapman SK, Hart SC, Cobb NS, Whitham TG, Koch GW (2003) Insect herbivory increases litter quality and decomposition: an extension of the acceleration hypothesis. Ecology 84:2867–2876
DeLoach CJ, Carruthers RI, Lovich JE, Dudley TL, Smith SD (2000) Ecological interactions in the biological control of saltcedar (Tamarix spp.) in the United States: toward a new understanding. In: Spencer NR (ed) Proceedings of the X international symposium on biological control of weeds. Montana State University, Bozeman, pp 819–873
Denslow JS, D’Antonio CM (2005) After biocontrol: assessing indirect effects of insect releases. Biol Control 35:307–318
Di Tomaso JM (1998) Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol 12:326–336
Dudley TL (2005) Progress and pitfalls in the biological control of saltcedar (Tamarix spp.) in North America. Proceedings of the 16th US Department of Agriculture interagency research forum on gypsy moth and other invasive species. GTR-NE-337
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Friedman JM, Auble GT, Shafroth PB, Scott ML, Merigliano MF, Preehling MD, Griffin EK (2005) Dominance of non-native riparian trees in western USA. Biol Invasions 7:747–751
Furutani SC, Arita LH, Fuji JK (1990) Relationship between simulated Chinese Rose Beetle (Coleoptera: Scarabaeidae) feeding and photosynthetis rate reduction. Proc Hawaiian Entomol Soc 30:97–104
Gaskin JF, Schaal BA (2002) Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proc Natl Acad Sci USA 99:11256–11259
Gaskin JF, Ghahremani-nejad F, Zhang DY, Londo JP (2004) A systematic overview of Frankeniaceae and Tamaricaceae from nuclear rDNA and plastid sequence data. Ann Mo Bot Gard 91:401–409
Glenn EP, Nagler PL (2005) Comparative ecophysiology of Tamarix ramosissima and native trees in western US riparian zones. J Arid Environ 61:419–446
Holmgren NH, Holmgren PK, Cronquist A (2005) Intermountain flora, vascular plants of the intermountain west, USA, volume 2, part B. The New York Botanical Garden, New York
Horton JL, Kolb TE, Hart SC (2001) Physiological response to groundwater depth varies among species and with river flow regulation. Ecol Appl 11:1046–1059
Hudgeons JL, Knutson AE, Heinz KM, DeLoach CJ, Dudley TL, Pattison RR, Kiniry JR (2007) Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera : Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae). Biol Control 43:213–221
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago
Kosola KR, Dickmann DI, Paul EA, Parry D (2001) Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129:65–74
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS© for mixed models, 2nd edn. SAS Institute Inc, Cary
Morisette JT, Jarnevich CS, Ullah A, Cai WJ, Pedelty JA, Gentle JE, Stohlgren TJ, Schnase JL (2006) A tamarisk habitat suitability map for the continental United States. Front Ecol Environ 4:11–17
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. J Biol Chem 153:375–380
Nobel PS, Alm DM, Cavelier J (1992) Growth respiration, maintenance respiration and structural- carbon costs for roots of 3 desert succulents. Funct Ecol 6:79–85
Norby RJ, Cotrufo MF, Ineson P, O’Neill EG, Canadell JG (2001) Elevated CO2, litter chemistry, and decomposition: a synthesis. Oecologia 127:153–165
Nowak RS, Caldwell MM (1984) A test of compensatory photosynthesis in the field—implications for herbivory tolerance. Oecologia 61:311–318
O’Neal ME, Landis DA, Isaacs R (2002) An inexpensive, accurate method for measuring leaf area and defoliation through digital image analysis. J Econ Entomol 95:1190–1194
Owens CS, Madsen JD (1998) Phenological studies of carbohydrate allocation in hydrilla. J Aquat Plant Manage 36:40–44
Raguse CA, Smith D (1965) Carbohydrate content in alfalfa herbage as influenced by methods of drying. J Agric Food Chem 13:306–309
Reich PB, Walters MB, Tjoelker MG, Vanderklein D, Buschena C (1998) Photosynthesis and respiration rates depend on leaf and root morphology and nitrogen concentration in nine boreal tree species differing in relative growth rate. Funct Ecol 12:395–405
Ritchie ME, Tilman D, Knops JMH (1998) Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology 79:165–177
Schowalter TD, Hargrove WW, Crossley DA (1986) Herbivory in forested ecosystems. Annu Rev Entomol 31:177–196
Schroder R, Forstreuter M, Hilker M (2005) A plant notices insect egg deposition and changes its rate of photosynthesis. Plant Physiol 138:470–477
Shain L, Hillis WE (1972) Ethylene Production in Pinus-Radiata in Response to Sirex-Amylostereum attack. Phytopathology 62:1407–1409
Snyder KA, Williams DG (2003) Defoliation alters water uptake by deep and shallow roots of Prosopis velutina (Velvet Mesquite). Funct Ecol 17:363–374
Snyder KA, Williams DG (2007) Root allocation and water uptake patterns in riparian tree saplings: responses to irrigation and defoliation. For Ecol Manage 246:222–231
Snyder KA, Richards JH, Donovan LA (2003) Night-time conductance in C-3 and C-4 species: do plants lose water at night? J Exp Bot 54:861–865
Stiling P, Simberloff D (1989) Leaf abscission-induced defense against pests or response to damage. Oikos 55:43–49
Swank JC, Below FE, Lambert RJ, Hageman RH (1982) Interaction of carbon and nitrogen-metabolism in the productivity of maize. Plant Physiol 70:1185–1190
Taylor JE, Whitelaw CA (2001) Signals in abscission. New Phytol 151:323–339
Tracy JL, Robbins TO (2009) Taxonomic revision and biogeography of the Tamarix-feeding Diorhabda elongata (Brullé, 1832) species group (Coleoptera: Chrysomelidae: Galerucinae: Galerucini) and analysis of their potential in biological control of Tamarisk. Zootaxa 2101:1–152
Uselman SM, Qualls RG, Lilienfein J (2007) Fine root production across a primary successional ecosystem chronosequence at Mt. Shasta, California. Ecosystems 10:703–717
Vandermeijden E, Wijn M, Verkaar HJ (1988) Defense and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Acknowledgments
We thank the Pyramid Lake Paiute Tribe for access to their lands. We also wish to thank the following USDA-ARS staff: Livy Williams III and Kirk Tonkel for providing the Diorhabda carinulata beetles used in the experiment; Brenda Grewell and Joy Futrell for determination of TNC concentrations; Delilah Wood and Tina Williams for providing the light microscopy and SEM images; and Tye Morgan and Bob Blank for their assistance with nutrient analyses. We are grateful to Amira Dittrich, Mike Schmeiske, and Sarah Thompson for valuable field and laboratory assistance. We thank Brenda Grewell, Lincoln Smith, and anonymous reviewers for comments on this manuscript. The experiments conducted in this study comply with the current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snyder, K.A., Uselman, S.M., Jones, T.J. et al. Ecophysiological responses of salt cedar (Tamarix spp. L.) to the northern tamarisk beetle (Diorhabda carinulata Desbrochers) in a controlled environment. Biol Invasions 12, 3795–3808 (2010). https://doi.org/10.1007/s10530-010-9772-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9772-1