Abstract
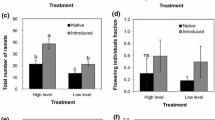
With the extensive spread of invasive species throughout North America and Europe there is an urgent need to better understand the morphological and physiological characteristics of successful invasive plants and the evolutionary mechanisms that allow introduced species to become invasive. Most ecological studies have focused on morphological differences and changes in community dynamics, and physiological studies have typically explored the differences between native and invasive species. In this study, 15 different genotypes of Phalaris arundinacea from both its native (European) and invasive (North American) range were grown in a common garden experiment to monitor the physiological differences between native and invasive genotypes. Here we present data that suggests high variability exists in the physiological traits among genotypes of P. arundinacea, yet genotypes from the native range are not necessarily physiologically inferior to the hybridized invasive genotypes. Previous work has shown that multiple introductions of P. arundinacea from various European locations to the United States resulted in numerous hybridization events, yielding more genetic variability and phenotypic plasticity in the invasive range. Of the genotypes studied, both morphological and physiological traits of genotypes with French origin were significantly different from the plants from the Czech Republic, North Carolina, and Vermont. The lack of clear differences between native and invasive genotypes indicates that physiological traits may be highly conserved in P. arundinacea and enhanced photosynthetic rates are not indicative of successful invasive genotypes. Instead, morphological traits and defensive secondary compound metabolism may play a more important role in the success of P. arundinacea within its invasive range, and patterns of genetic variation in physiological traits between invasive and native range may be more important than the mean traits of each region when explaining reed canarygrass’ invasive potential in North America.



Similar content being viewed by others
References
Amsellem L, Noyer J-L, Hossaert-McKey M (2001) Evidence for a switch in the reproductive biology of Rubus alceifolius (Rosaceae) towards apomixis, between its native range and its area of introduction. Am J Bot 88:2243–2251
Arntz MA, Delph LF (2004) Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologia 127(4):455–467
Baskauf CJ, Eickmeier WG (1994) Comparative ecophysiology of a rare and a widespread species of Echinacea (Asteraceae). Am J Bot 81:958–964
Benowicz A, Guy RD, El-Kassaby YA (2000) Geographic pattern of genetic variation in photosynthetic capacity and growth in two hardwood species from British Columbia. Oecologia 123:168–174
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol 83:887–889
Bohn T, Sandlund OT, Amundsen P, Primicerio R (2004) Rapidly changing life history during invasion. Oikos 106:138–150
Bonnin I, Prosperi JM, Olivieri I (1996) Genetic markers and quantitative genetic variation in Medicago truncatula (Leguminosae): a comparative analysis of population structure. Genetics 143:1795–1805
Brown JS, Eckert CG (2005) Evolutionary increase in sexual and clonal reproductive capacity during biological invasion in an aquatic plant Butomus umbellatus (Butomaceae). Am J Bot 92:495–502
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Callaway R, Maron JL (2006) What have exotic plant invasions taught us over the past 20 years? Trends Ecol Evol 21:369–374
Chapin FSI, Oechel WC (1983) Photosynthesis, respiration, and phosphate absorption by Carex aquatilis ecotypes along latitudinal and local environmental gradients. Ecology 64:743–751
Cruz C, Lips H, Martins-Loução MA (2003) Nitrogen use efficiency by a slow-growing species as affected by CO2 levels, root temperature, N source and availability. J Plant Physiol 160(12):1421–1428
DeWalt SJ, Denslow JS, Hamrick JL (2004) Biomass allocation, growth, and photosynthesis of genotypes from native and introduced ranges of the tropical shrub Clidemia hirta. Oecologia 138:521–531
Dijkstra P, Lambers H (1989) Analysis of specific leaf area and photosynthesis of two inbred lines of Plantago major differing in relative growth rate. New Phytol 113:283–290
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Feng Y, Wang J, Sang W (2007) Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecologica 31:40–47
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Grime JP (1977) Evidence for three primary strategies in plant and its relevance to ecological and evolutionary theory. Am Nat 111:1169–1194
Grime JP, Hunt R (1975) Relative growth-rate: its range and adaptative significance in a local flora. J Ecol 63:393–422
Heschel MS, Stinchcombe JR, Holsinger KE, Schmitt J (2004) Natural selection on light response curve parameters in the herbaceous annual, Impatiens capensis. Oecologia 139:487–494
Houle D (1992) Comparing evolvability and variability of quantitative traits. Genetics 130:195–204
Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L (2000) Rapid evolution of a geographic cline in size in an introduced fly. Science 287:308–309
Körner C, Diemer M (1987) In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct Ecol 1:179–194
Lafuma L, Maurice S (2007) Increase in mate availability without loss of self-incompatibility in the invasive species Senecio inaequidens (Asteraceae). Oikos 116:201–208
Lambrinos JL (2004) How interactions between ecology and evolution influence contemporary invasion dynamics. Ecology 85:2061–2070
Lavergne S, Molofsky J (2004) Reed canary grass (Phalaris arundinacea L.), as a biological model in the study of plant invasions. Crit Rev Plant Sci 23:415–429
Lavergne S, Molofsky J (2006) Control strategies for the invasive reed canarygrass (Phalaris arundinacea L.) in North American wetlands: the need for an integrated management plan. Nat Areas J 26:208–214
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA 104:3883–3888
Mack RN (1995) Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol Conserv 78(1):107–121
Maron JL (2006) The relative importance of latitude matching and propagule pressure in the colonization success of an invasive forb. Ecography 29:819–826
Maron JL, Vilà M, Bommarco R, Elmendorf S,Beardsley P (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
Massei G, Hartley SE, Bacon PJ (2000) Chemical and morphological variation of Mediterranean woody evergreen species: Do plants respond to ungulate browsing? J Veg Sci 11(1):1–8
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89:1431–1438
Mooney HA, Billings WD (1961) Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecol Monogr 31:1–29
Peek MS, Russek-Cohen E, Wait DA, Forseth IN (2002) Physiological response curve analysis using nonlinear mixed models. Oecologia 132:175–180
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer-Verlag, New York
Poorter H, Garnier E (1999) Ecological significance of inherent variation in relative growth rate and its components. In: Pugnaire FI, Valladore F (eds) Handbook of functional plant ecology. Marcel Dekker Inc, New York, pp 81–103
Potvin C, Lechowicz MJ, Tardif S (1990) The statistical analysis of ecophysiological response curves obtained from expriments involving repeated measures. Ecology 71:1389–1400
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94:13730–13734
Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80:1955–1969
Roff DA (1997) Evolutionary quantitative genetics. Chapman and Hall, New York, 493 pp
Siemann E, Rogers WE (2001) Genetic differences in growth of an invasive tree species. Ecol Lett 4:514–518
Stockwell CA, Hendry AP, Kinnison MT (2003) Contemporary evolution meets conservation biology. Trends Ecol Evol 18:94–10
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, Berlin
Zangerl AR, Bazzaz FA (1983) Plasticity and genotypic variation in photosynthetic behavior of an early and late successional species of Polygonum. Oecologia 57:270–273
Acknowledgements
The authors would like to thank Dr. Paul Schaberg for the use of a LI-6400 unit. This research was supported by U.S. Department of Agriculture (USDA) Hatch and USDA Cooperative Research, Education, and Extension Service Award 2003-35320-13503 (to J.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brodersen, C., Lavergne, S. & Molofsky, J. Genetic variation in photosynthetic characteristics among invasive and native populations of reed canarygrass (Phalaris arundinacea). Biol Invasions 10, 1317–1325 (2008). https://doi.org/10.1007/s10530-007-9206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9206-x