Abstract
Objective
The effects of a brief (3 days) and prolonged (6 days) period of incubation in darkness and light on the biomass content, lipid content and fatty acid profile in Chlorella vulgaris UMT-M1 were determined.
Results
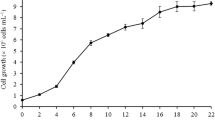
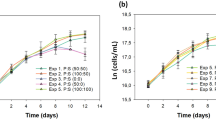
Three days of incubation in darkness increased saturated fatty acid (SFA) content from 34.0 to 41.4% but decreased monounsaturated fatty acid (MUFA) content from 36.7 to 29.8%. Palmitic acid (C16:0) content was increased from 23.2 to 28.9%, whereas oleic acid (C18:1) content was reduced from 35.4 to 28.8%. Total oil content was slightly decreased from 20.4 to 18.7% after 3 days of darkness, without a significant reduction in biomass compared to 3 days of incubation in light. Biomass and oil content was highest in cultures incubated for 6 days in light, however the stimulatory and inhibitory effects of darkness (or light) on SFA and MUFA content was no longer present at 6 days of incubation.
Conclusions
Findings from this study suggests that fatty acid composition in C. vulgaris could be modulated to favor either C16:0 or C18:1 by a brief period of either darkness or light incubation, prior to harvesting.




Similar content being viewed by others
References
Álvarez-Díaz PD, Ruiz J, Arbib Z, Barragán J, Garrido-Pérez C, Perales JA (2014) Lipid production of microalga Ankistrodesmus falcatus increased by nutrient and light starvation in a two-stage cultivation process. Appl Biochem Biotechnol 174:1471–1483
Anne-Marie K, Yee W, Loh SH, Aziz A, Cha TS (2020) Effects of excess and limited phosphate on biomass, lipid and fatty acid contents and the expression of four fatty acid desaturase genes in the tropical Selenastraceaen Messastrum gracile SE-MC4. Appl Biochem Biotechnol 190:1438–1456
Bai X, Song H, Lavoie M, Zhu K, Su Y, Ye H, Chen S, Fu Z, Qian H (2016) Proteomic analyses bring new insights into the effect of a dark stress on lipid biosynthesis in Phaeodactylum tricornutum. Sci Rep 6:25494
Borges L, Morón-Villarreyes JA, D’Oca MG, Abreu PC (2011) Effects of flocculants on lipid extraction and fatty acid composition of the microalgae Nannochloropsis oculata and Thalassiosira weissflogii. Biomass Bioenergy 35(10):4449–4454
Brányiková I, Maršálková B, Doucha J, Brányik T, Bišová K, Zachleder V, Vítová M (2011) Microalgae-novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Cha TS, Chen JW, Goh EG, Aziz A, Loh SH (2011) Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour Technol 102:10633–10640
Cha TS, Chee JY, Loh SH, Jusoh M (2018) Oil production and fatty acid composition of Chlorella vulgaris cultured in nutrient-enriched solid-agar-based medium. Bioresour Technol Rep 3:218–223
Chen Y, Wang J, Zhang W, Chen L, Gao L, Liu T (2013) Forced light/dark circulation operation of open pond for microalgae cultivation. Biomass Bioenergy 56:464–470
Chen T, Liu J, Guo B (2015) Light attenuates lipid accumulation while enhancing cell proliferation and starch synthesis in the glucose-fed oleaginous microalga Chlorella zofingiensis. Sci Rep 5:14936
Chi NT, Duc PA, Mathimani T, Pugazhendhi A (2019) Evaluating the potential of green alga Chlorella sp. for high biomass and lipid production in biodiesel viewpoint. Biocatal Agric Biotechnol 17:184–188
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Choi GG, Kim BH, Ahn CY, Oh HM (2011) Effect of nitrogen limitation on oleic acid biosynthesis in Botryococcus braunii. J Appl Phycol 23:1031–1037
Damiani MC, Popovich CA, Constenla D, Leonardi PI (2010) Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol 101:3801–3807
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feedstocks. Appl Energy 102:1371–1381
Doucha J, Lívanský K (2006) Productivity, CO2/O2 exchange and hydraulics in outdoor open high density microalgal (Chlorella sp.) photobioreactors operated in a Middle and Southern European climate. J Appl Phycol 18:811–826
Edmundson SJ, Huesemann MH (2015) The dark side of algae cultivation: characterizing night biomass loss in three photosynthetic algae, Chlorella sorokiniana, Nannochloropsis salina and Picochlorum sp. Algal Res 12:470–476
Gim GH, Kim JK, Kim HS, Kathiravan MN, Yang H, Jeong SH, Kim SW (2014) Comparison of biomass production and total lipid content of freshwater green microalgae cultivated under various culture conditions. Bioproc Biosyst Eng 37:99–106
Gim GH, Ryu J, Kim MJ, Kim PI, Kim SW (2016) Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J Ind Microbiol Biotechnol 43:605–616
Griffiths MJ, van Hille RP, Harrison ST (2012) Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J Appl Phycol 24:989–1001
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms I. Cyclotella nana hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8:229–239
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Jeong H, Lee J, Cha M (2013) Energy efficient growth control of microalgae using photobiological methods. Renew Energy 54:161–165
Khoeyi ZA, Seyfabadi J, Ramezanpour Z (2012) Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac Int 20:41–49
Knothe G (2009) Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci 2:759–766
Lau CC, Loh SH, Aziz A, Cha TS (2017) Effects of disrupted omega-3 desaturase gene construct on fatty acid composition and expression of four fatty acid biosynthetic genes in transgenic Chlorella vulgaris. Algal Res 26:143–152
Liao Q, Li L, Chen R, Zhu X (2014) A novel photobioreactor generating the light/dark cycle to improve microalgae cultivation. Bioresour Technol 161:186–191
Liu J, Yuan C, Hu G, Li F (2012) Effects of light intensity on the growth and lipid accumulation of microalga Scenedesmus sp. 11–1 under nitrogen limitation. Appl Biochem Biotechnol 166:2127–2137
Lv X, Zou L, Sun B, Wang J, Sun MY (2010) Variations in lipid yields and compositions of marine microalgae during cell growth and respiration, and within intracellular structures. J Exp Mar Biol Ecol 391:73–83
Maksimova IV, Bratkovskaya LB, Plekhanov SE (2004) Extracellular carbohydrates and polysaccharides of the alga Chlorella pyrenoidosa Chick S-39. Biol Bull 31:175–181
Mock T, Kroon BM (2002) Photosynthetic energy conversion under extreme conditions—II: the significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry 61:53–60
Nzayisenga JC, Farge X, Groll SL, Sellstedt A (2020) Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol Biofuels 13:4
Ogbonna JC, Tanaka H (1996) Night biomass loss and changes in biochemical composition of cells during light/dark cyclic culture of Chlorella pyrenoidosa. J Ferment Bioeng 82:558–564
Poh ZL, Kadir WN, Lam MK, Uemura Y, Suparmaniam U, Lim JW, Show PL, Lee KT (2020) The effect of stress environment towards lipid accumulation in microalgae after harvesting. Renew Energy 154:1083–1091
Rizwan M, Mujtaba G, Memon SA, Lee K (2020) Influence of salinity and nitrogen in dark on Dunaliella tertiolecta’s lipid and carbohydrate productivity. Biofuels. https://doi.org/10.1080/17597269.2020.1762275
Seyfabadi J, Ramezanpour Z, Khoeyi ZA (2011) Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J Appl Phycol 23:721–726
Sforza E, Simionato D, Giacometti GM, Bertucco A, Morosinotto T (2012) Adjusted light and dark cycles can optimize photosynthetic efficiency in algae growing in photobioreactors. PLoS ONE 7:e38975
Simionato D, Sforza E, Carpinelli EC, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresour Technol 102:6026–6032
Sun X, Cao Y, Xu H, Liu Y, Sun J, Qiao D, Cao Y (2014) Effect of nitrogen starvation, light intensity and iron on triacylglyceride/carbohydrate production and fatty acid profile of Neochloris oleoabundans HK-129 by a two-stage process. Bioresour Technol 155:204–212
Sutherland DL, Park J, Heubeck S, Ralph PJ, Craggs RJ (2020) Size matters–Microalgae production and nutrient removal in wastewater treatment high rate algal ponds of three different sizes. Algal Res 45:101734
Teh KY, Afifudeen CW, Aziz A, Wong LL, Loh SH, Cha TS (2019) De novo whole genome sequencing data of two mangrove-isolated microalgae from Terengganu coastal waters. Data Brief 27:104680
Xia L, Li Y, Huang R, Song S (2017) Effective harvesting of microalgae by coagulation–flotation. R Soc Open Sci 4:170867
Yee W, Tang SG, Phua PS, Megawarnan H (2019) Long-term maintenance of 23 strains of freshwater microalgae on solid microbiological culture media: a preliminary study. Algal Res 41:101516
Zhukova NV (2007) Changes in the fatty acid composition of symbiotic dinoflagellates from the hermatypic coral Echinopora lamellosa during adaptation to the irradiance level. Russ J Plant Physiol 54:763–769
Acknowledgements
This research project was supported by the Ministry of Higher Education under the Fundamental Research Grant Scheme (FRGS FASA 1-2010: VOT 59175). The authors would like to thank Miss Teng Fei Chien for her kind help during the project period.
Supporting information
Supplementary Table 1—Biomass dry weight and total oil content of C. vulgaris cultures under a brief (3 day) and prolonged (6 day) period of light and dark treatments.
Supplementary Table 2—Saturated, monounsaturated and polyunsaturated fatty acids of C. vulgaris cultures under a brief (3 day) and prolonged (6 day) period of light and dark treatments.
Supplementary Table 3—Composition of fatty acids produced by C. vulgaris under a brief (3 day) and prolonged (6 day) period of light and dark treatments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cha, T.S., Yee, W., Phua, P.S.P. et al. A brief period of darkness induces changes in fatty acid biosynthesis towards accumulation of saturated fatty acids in Chlorella vulgaris UMT-M1 at stationary growth phase. Biotechnol Lett 43, 803–812 (2021). https://doi.org/10.1007/s10529-021-03077-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03077-2