Abstract
Objectives
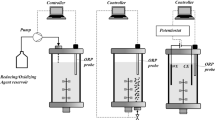
A redox potential-driven fermentation, maintaining dissolved oxygen at a prescribed level while simultaneously monitoring the changes of fermentation redox potential, was developed to guide the cultivation progress of recombinant protein expression.
Results
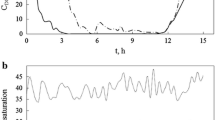
A recombinant E. coli harboring prolinase-expressing plasmid (pKK-PepR2) was cultivated using the developed process. Two distinct ORP valleys were noticeable based on recorded profile. The first ORP valley is equivalent to the timing for the addition of inducing agent, and the second ORP valley serves to guide the timing for cell harvesting. The final prolinase activity is 0.172 μmol/mg/min as compared to that of 0.154 μmol/mg/min where the optical density was employed to guide the timing of inducer addition and an empirically determined length of the cultivation.
Conclusion
The developed process can be further modified to become an automatic operation.

Similar content being viewed by others
Abbreviations
- DO:
-
Dissolved oxygen
- IPTG:
-
Isopropyl β-d-1-thiogalactopyranoside
- OD:
-
Optical density
- ORP:
-
Fermentation redox potential
References
Cirkovas A, Sereikaite J (2010) Increase in the solubility of recombinant mink growth hormone at low cultivation temperature of E. coli. Biotechnol Biotechnol Equip 24:2169–2171
Dahod SK (1982) Redox potential as a better substitute for dissolved oxygen in fermentation process control. Biotechnol Bioeng 24:2123–2125
Harms J, Wang X, Kim T, Yang X, Tathore A (2008) Defining process design space for biotech products: case study of Pichia pastoris fermentation. Biotechnol Prog 24:655–662
Huang Y, Tanaka T (2015) Characterization of two putative prolinases (PepR1 and PepR2) from Lactobacillus plantarum WCFS1: occurrence of two isozymes with structural similarity and different catalytic properties. Biochim Biophys Acta Proteins Proteom 1854:91–100
Lin YH, Chien WS, Duan KJ (2010) Correlations between reduction–oxidation potential profiles and growth patterns of Saccharomyces cerevisiae during very-high-gravity fermentation. Process Biochem 45:765–770
Liu CG, Qin JC, Lin YH (2017) Fermentation processes. In: Jozala A (ed) Fermentation and redox potential. IntechOpen, London, pp 23–42
Ou J, Wang L, Ding X, Du J, Zhang Y, Chen H, Xu A (2004) Stationary phase protein overproduction is a fundamental capability of Escherichia coli. Biochem Biophys Res Res 314:174–180
San-Miguel T, Pérez-Bermúdez P, Gavidia I (2013) Production of soluble eukaryotic recombinant proteins in E. coli is favoured in early log-phase cultures induced at low temperature. Springerplus 2:1–4
Stevenson K, McVey A, Clark I, Swain P, Pilizota T (2016) General calibration of microbial growth in microplate readers. Sci Rep 6:38828
Funding
The authors acknowledge the financial support from the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, J., Wu, Y., Tanaka, T. et al. Development of redox potential-driven fermentation process for recombinant protein expression. Biotechnol Lett 43, 99–103 (2021). https://doi.org/10.1007/s10529-020-03030-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03030-9