Abstract
Objectives
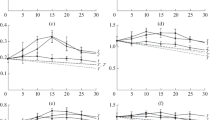
The synergistic effects between cellulases and lytic polysaccharide monooxygenases (LPMOs) were investigated systematically in terms of their degree of synergy (DS) on amorphous and crystalline cellulose. Synergy curves were obtained for enzyme pairs containing a cellulase from Trichoderma reesei (Cel6A and Cel7A) and three LPMOs from Thermoascus aurantiacus (TaAA9A), Lentinus similis (LsAA9A) and Thielavia terrestris (TtAA9E).
Results
The synergistic experiments showed that the three LPMOs significantly improved the hydrolytic efficiency of Cel6A, on both cellulosic substrates; a more pronounced effect being seen for TtAA9E on amorphous cellulose at low cellulase:LPMO ratios. In contrast, the highly processive, reducing-end acting Cel7A synergised with the C1-C4 oxidising LPMOs, TaAA9A and LsAA9A, but was inhibited by the presence of C1-oxidizing TtAA9E.
Conclusions
The degree of synergy exhibited by the cellulase-LPMO mixtures was enzyme- and substrate-specific. The observed Cel7A inhibition, rather than synergy, by the C1-oxidizing LPMO, TtAA9E, warrants further investigations.



Similar content being viewed by others
References
Andersen N, Johansen KS, Michelsen M et al (2008) Hydrolysis of cellulose using mono-component enzymes shows synergy during hydrolysis of phosphoric acid swollen cellulose (PASC), but competition on Avicel. Enzyme Microb Technol 42:362–370
Badino SF, Christensen SJ, Kari J et al (2017) Exo-exo synergy between Cel6A and Cel7A from Hypocrea jecorina: Role of carbohydrate binding module and the endo-lytic character of the enzymes. Biotechnol Bioeng 114:1639–1647
Boisset C, Pétrequin C, Chanzy H et al (2001) Optimized Mixtures of Recombinant Humicola insolens Cellulases for the Biodegradation of Crystalline Cellulose Claire. Biotechnol Bioeng 72:339–345
Cannella D, Hsieh CC, Felby C et al (2012) Production and effect of aldonic acids during enzymatic hydrolysis of lignocellulose at high dry matter content. Biotechnol Biofuels 5:1–10
Christensen SJ, Kari J, Badino SF et al (2018) Rate-limiting step and substrate accessibility of cellobiohydrolase Cel6A from Trichoderma reesei. FEBS J 285:4482–4493
Eibinger M, Ganner T, Bubner P et al (2014) Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J Biol Chem 289:35929–35938
Frandsen KEH, Simmons TJ, Dupree P et al (2016) The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat Chem Biol 12:298–303
Harris PV, Welner D, McFarland KC et al (2010) Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry 49:3305–3316
Hu J, Arantes V, Pribowo A et al (2014) Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ Sci 7:2308–2315
Jalak J, Kurašin M, Teugjas H, Väljamäe P (2012) Endo-exo Synergism in Cellulose Hydrolysis Revisited. J Biol Chem 287:28802–28815
Johansen KS (2016) Discovery and industrial applications of lytic polysaccharide mono-oxygenases. Biochem Soc Trans 44:143–149
Kari J, Christensen SJ, Andersen M et al (2019) A practical approach to steady-state kinetic analysis of cellulases acting on their natural insoluble substrate. Anal Biochem 586:113411
Klyosov AA (1990) Trends in Biochemistry and Enzymology of Cellulose Degradation. Biochemistry 29:10577–10585
Kracher D, Andlar M, Furtmüller PG, Ludwig R (2018) Active-site copper reduction promotes substrate binding of fungal lytic polysaccharide monooxygenase and reduces stability. J Biol Chem 293:1676–1687
Lever M (1973) Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med 7:274–281
Mandels M, Reese ET (1965) Inhibition of cellulases. Annu Rev Phytopathol 3:85–102
Müller G, Várnai A, Johansen KS et al (2015) Harnessing the potential of LPMO-containing cellulase cocktails poses new demands on processing conditions. Biotechnol Biofuels 8:1–9
Müller G, Chylenski P, Bissaro B et al (2018) The impact of hydrogen peroxide supply on LPMO activity and overall saccharification efficiency of a commercial cellulase cocktail. Biotechnol Biofuels 11:1–17
Olsen JP, Borch K, Westh P (2017) Endo/exo-synergism of cellulases increases with substrate conversion. Biotechnol Bioeng 114:696–700
Quinlan RJ, Sweeney MD, Lo Leggio L et al (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc Natl Acad Sci 108:15079–15084
Scott BR, Zhi H, Jesper H et al (2016) Catalase improves saccharification of lignocellulose by reducing lytic polysaccharide monooxygenase-associated enzyme inactivation. Biotechnol Lett 38:425–434
Simmons TJ, Frandsen KEH, Ciano L et al (2017) Structural and electronic determinants of lytic polysaccharide monooxygenase reactivity on polysaccharide substrates. Nat Commun 8(1):1–12
Singh RK, Möllers KB, Russo DA et al (2019) Thermal Unfolding and Refolding of Lytic Polysaccharide Monooxygenase. R Soc Chem Adv 9(51):29734–29742
Song B, Li B, Wang X et al (2018) Real-time imaging reveals that lytic polysaccharide monooxygenase promotes cellulase activity by increasing cellulose accessibility. Biotechnol Biofuels 11:1–11
Väljamäe P, Sild V, Nutt A et al (1999) Acid hydrolysis of bacterial cellulose reveals different modes of synergistic action between cellobiohydrolase I and endoglucanase I. Eur J Biochem 266:327–334
Vermaas JV, Crowley MF, Beckham GT, Payne CM (2015) Effects of lytic polysaccharide monooxygenase oxidation on cellulose structure and binding of oxidized cellulose oligomers to cellulases. J Phys Chem B 119:6129–6143
Westh P, Kari J, Olsen J et al (2014) Cellobiohydrolase variants and polynucleotides encoding same. 27587
Xu F, Ding H, Tejirian A (2009) Detrimental effect of cellulose oxidation on cellulose hydrolysis by cellulase. Enzyme Microb Technol 45:203–209
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: Noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824
Zhou S, Ingram LO (2000) Synergistic hydrolysis of carboxymethyl cellulose and acid-swollen cellulose by two endoglucanases (CelZ and CelY) from Erwinia chrysanthemi. J Bacteriol 182:5676–5682
Zhou H, Li T, Yu Z et al (2019) A lytic polysaccharide monooxygenase from Myceliophthora thermophila and its synergism with cellobiohydrolases in cellulose hydrolysis. Int J Biol Macromol 139:570–576
Acknowledgements
This work was supported by the Novo Nordisk Foundation, Grant NO. NNF17SA0027704 to Katja Salomon Johansen.
Supporting information: Supplementary Fig. 1 – Comparison of cellulose degradation fingerprint of TaAA9A, LsAA9A and TtAA9E.
Supplementary material
Supplementary experiments were performed, in order to confirm the purity of the three LPMO enzyme preparations, as well as to compare directly their activity. Supplementary Fig. 1 shows a direct comparison of the activity of the three tested LPMOs – TaAA9A, LsAA9A and TtAA9E. PASC degradation experiments were carried out in 50 mM Citrate/Phosphate buffer (pH 6), with copper-loaded LPMO at a concentration of 4 µM, 0.4 g/L PASC and 4 mM ascorbic acid. Samples were incubated at 50 °C for 24 h in an Eppendorf Thermomixer at 1100 rpm. The reaction mixture was centrifuged through filter plates with 0.22 µm pore size (Millipore MultiScreenHTS, 3 min, 3500 rpm) and the supernatant was analysed by HPAEC-PAD.
TtAA9E’s strict C1-oxidation mechanism is confirmed by the lack of C4-oxidised oligosaccharides on the chromatogram (after 25 min). The proposed substrate-specific regioselectivity of LsAA9A can be observed by the presence of C4- and absence of C1-oxidised sugars. LsAA9A’s ability to cleave short-chain oligosaccharides is also evident by the presence of a larger glucose peak, compared to the other two LPMOs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This article does not describe any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tokin, R., Ipsen, J.Ø., Westh, P. et al. The synergy between LPMOs and cellulases in enzymatic saccharification of cellulose is both enzyme- and substrate-dependent. Biotechnol Lett 42, 1975–1984 (2020). https://doi.org/10.1007/s10529-020-02922-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02922-0