Abstract
Objectives
To characterize a glycosyltransferase (UGT74AN3) from Catharanthus roseus and investigate its specificity toward cardiotonic steroids and phenolic compounds.
Results
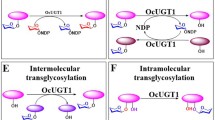
UGT74AN3, a novel permissive GT from C. roseus, displayed average high conversion rate (> 90%) toward eight structurally different cardiotonic steroids. Among them, resibufogenin, digitoxigenin, and uzarigenin gave 100% yield. Based on LC–MS, 1H-NMR and 13C-NMR analysis, structure elucidation of eight glycosides was consistent with 3-O-β-d-glucosides. We further confirmed UGT74AN3 was permissive enough to glycosylate curcumin, resveratrol, and phloretin. The cDNA sequence of UGT74AN3 contained an ORF of 1,425 nucleotides encoding 474 amino acids. UGT74AN3 performed the maximum catalytic activity at 40 °C, pH 8.0, and was divalent cation-independent. Km values of UGT74AN3 toward resibufogenin, digitoxigenin, and uzarigenin were 7.0 µM, 12.3 µM, and 17.4 µM, respectively.
Conclusions
UGT74AN3, a glycosyltransferase from a noncardenolide-producing plant, displayed catalytic efficiency toward cardiotonic steroids and phenolic compounds, which would make it feasible for glycosylation of bioactive molecules.



Similar content being viewed by others
References
Beale TM, Taylor MS (2013) Synthesis of cardiac glycoside analogs by catalyst-controlled, regioselective glycosylation of digitoxin. Org Lett 15:1358–1361
Brazier-Hicks M, Edwards LA, Edwards R (2007) Selection of plants for roles in phytoremediation: the importance of glucosylation. Plant Biotechnol J 5:627–635
Dai L, Liu C, Zhu Y, Zhang J, Men Y, Zeng Y, Sun Y (2015) Functional characterization of cucurbitadienol synthase and triterpene glycosyltransferase involved in biosynthesis of mogrosides from Siraitia grosvenorii. Plant Cell Physiol 56:1172–1182
Gantt RW, Peltier-Pain P, Thorson JS (2011) Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat Prod Rep 28:1811–1853
Iyer AK, Zhou M, Azad N, Elbaz H, Wang L, Rogalsky DK, Rojanasakul Y, O'Doherty GA, Langenhan JM (2010) A direct comparison of the anticancer activities of digitoxin MeON-neoglycosides and O-glycosides: oligosaccharide chain length-dependent induction of caspase-9-mediated apoptosis. ACS Med Chem Lett 1:326–330
Jung SC, Kim W, Park SC, Jeong J, Park MK, Lim S, Lee Y, Im WT, Lee JH, Choi G, Kim SC (2014) Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol 55:2177–2188
Kaminaga Y, Sahin FP, Mizukami H (2004) Molecular cloning and characterization of a glucosyltransferase catalyzing glucosylation of curcumin in cultured Catharanthus roseus cells. FEBS Lett 567:197–202
Li K, Feng J, Kuang Y, Song W, Zhang M, Ji S, Qiao X, Ye M (2017) Enzymatic synthesis of bufadienolide O-glycosides as potent antitumor agents using a microbial glycosyltransferase. Adv Synth Catal 359:3765–3772
Masada S, Terasaka K, Oguchi Y, Okazaki S, Mizushima T, Mizukami H (2009) Functional and structural characterization of a flavonoid glucoside 1,6-glucosyltransferase from Catharanthus roseus. Plant Cell Physiol 50:1401–1415
Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, Kroemer G, Galluzzi L (2013) Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology 2:e23082
Miettinen K, Dong L, Navrot N, Schneider T et al (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Commun 5:3606
Oguchi Y, Masada S, Kondo T, Terasaka K, Mizukami H (2007) Purification and characterization of UDP-glucose: curcumin glucoside 1,6-glucosyltransferase from Catharanthus roseus cell suspension cultures. Plant Cell Physiol 48:1635–1643
Piovan A, Cozza G, Caniato R, Moro S, Filippini R (2010) A novel glucosyltransferase from Catharanthus roseus cell suspensions. Process Biochem 45:655–659
Ramirez-Estrada K, Castillo N, Lara JA, Arró M, Boronat A, Ferrer A, Altabella T (2017) Tomato UDP-glucose sterol glycosyltransferases: a family of developmental and stress regulated genes that encode cytosolic and membrane-associated forms of the enzyme. Front Plant Sci 8:984
Tiwari P, Sangwan RS, Sangwan NS (2016) Plant secondary metabolism linked glycosyltransferases: an update on expanding knowledge and scopes. Biotechnol Adv 34:714–739
Wen C, Huang W, Zhu X, Li X, Zhang F, Jiang R (2018) UGT74AN1, a permissive glycosyltransferase from Asclepias curassavica for the regiospecific steroid 3-O-glycosylation. Org lett 20:534–537
Xie K, Chen R, Li J, Wang R, Chen D, Dou X, Dai J (2014) Exploring the catalytic promiscuity of a new glycosyltransferase from Carthamus tinctorius. Org Lett 16:4874–4877
Ye M, Dai J, Guo H, Cui Y, Guo D (2002) Glucosylation of cinobufagin by cultured suspension cells of Catharanthus roseus. Tetrahedron Lett 43:8535–8538
Ye T, Song W, Zhang J-J, An M, Feng S, Yan S, Li J (2017) Identification and functional characterization of DzS3GT, a cytoplasmic glycosyltransferase catalyzing biosynthesis of diosgenin 3-O-glucoside in Dioscorea zingiberensis. Plant Cell Tiss Org 129:399–410
Zhou M, Hou Y, Hamza A, Pain C, Zhan CG, Bugni TS, Thorson JS (2012) Probing the regiospecificity of enzyme-catalyzed steroid glycosylation. Org Lett 14:5424–5427
Acknowledgements
The study was financially supported by the Doctor Fundation of Jinggangshan University, China (No. JZB1821).
Supporting information
Section 1—MS, 1H NMR, 13C NMR data of compound 1a–8a.
Supplementary Table 1—The Genebank accession numbers and the primers of CrGT1-5.
Supplementary Table 2—Genebank accession numbers of the GTs used for phylogenetic analysis.
Supplementary Fig. 1—Alignment of five glycosyltransferases from Catharanthus roseus with UGT74AN1.
Supplementary Fig. 2—HPLC chromatogram of UGT74AN3 enzymatic products of bufadienolide aglycons.
Supplementary Fig. 3—HPLC chromatogram of UGT74AN3 enzymatic products of cardenolides aglycons.
Supplementary Fig. 4—HPLC chromatogram of UGT74AN3 enzymatic products of curcumin (9), resveratrol (10), and phloretin (11).
Supplementary Fig. 5—Effects of temperature (A), pH (B), divalent metal ions (C) and reaction time (D) on the activity of UGT74AN3.
Supplementary Fig. 6—Determination of kinetic parameters for UGT74AN3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, C., Huang, W., He, MM. et al. Cloning and characterization of a glycosyltransferase from Catharanthus roseus for glycosylation of cardiotonic steroids and phenolic compounds. Biotechnol Lett 42, 135–142 (2020). https://doi.org/10.1007/s10529-019-02756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02756-5