Abstract
Objective
We aimed to investigate the expression of a novel small cysteine-rich (SCR) effector protein SCR96 from the phytopathogenic oomycete Phytophthora cactorum in mammalian cells, its bioactivity and to exploit its polyclonal antibody.
Results
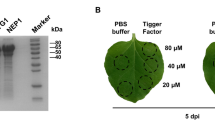
The gene encoding the SCR effector protein SCR96 was codon-optimized, custom-synthesized, cloned into pcDNA3.1(−) and overexpressed in human embryonic kidney (HEK) 293-6E cells. The recombinant protein SCR96 was prone to aggregation and purified with its monomer to homogeneity with a predicted molecular weight of 8.9 kDa. SCR96 exhibited strong phytotoxic activity on tomato seedlings at 24 h post treatment with 4.2 μg of the purified protein. An anti-SCR96 polyclonal antibody was prepared by immunization of New Zealand white rabbits. The good-titer antibody had a detection sensitivity at 6.25-ng level and could specifically detect the SCR96 protein expressed either in yeast, or in tomato leaves.
Conclusions
Transient production of the SCR effector protein SCR96 in mammalian cells is reliable, providing sufficient recombinant protein that can be utilized for analysis of its phytotoxic activity and preparation of its polyclonal antibody.






Similar content being viewed by others
References
Chen XR, Li YP, Li QY, Xing YP, Liu BB, Tong YH, Xu JY (2016) SCR96, a small cysteine-rich secretory protein of Phytophthora cactorum, can trigger cell death in the Solanaceae and is important for pathogenicity and oxidative stress tolerance. Mol Plant Pathol 17:577–587
Chen XR, Zhang Y, Li HY, Zhang ZH, Sheng GL, Li YP, Xing YP, Huang SX, Tao H, Kuan T, Zhai Y, Ma W (2019) The RXLR effector PcAvh1 is required for full virulence of Phytophthora capsici. Mol Plant Microbe Interact 32:986–1000
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. American Phytopathological Society, St. Paul
Fass D (2012) Disulfide bonding in protein biophysics. Annu Rev Biophys 41:63–79
Kamoun S (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44:41–60
Nicastro G, Orsomando G, Ferrari E, Manconi L, Desario F, Amici A, Naso A, Carpaneto A, Pertinhez TA, Ruggieri S, Spisni A (2009) Solution structure of the phytotoxic protein PcF: the first characterized member of the Phytophthora PcF toxin family. Protein Sci 18:1786–1791
Orsomando G, Lorenzi M, Raffaelli N, Dalla Rizza M, Mezzetti B, Ruggieri S (2001) Phytotoxic protein PcF, purification, characterization, and cDNA sequencing of a novel hydroxyproline-containing factor secreted by the strawberry pathogen Phytophthora cactorum. J Biol Chem 276:21578–21584
Orsomando G, Brunetti L, Pucci K, Ruggeri B, Ruggieri S (2011) Comparative structural and functional characterization of putative protein effectors belonging to the PcF toxin family from Phytophthora spp. Protein Sci 20:2047–2059
Schirrmann T, Büssow K (2010) Transient production of scFv-Fc fusion proteins in mammalian cells. In: Kontermann R, Dübel S (eds) Antibody Engineering. Springer, Heidelberg, pp 387–398
Schirrmann T, Al-Halabi L, Dübel S, Hust M (2008) Production systems for recombinant antibodies. Front Biosci 13:4576–4594
Song J (2007) Functional characterization of extracellular protease inhibitors of Phytophthora spp. and their targets tomato proteases. Dissertation, Ohio State University
Stergiopoulos I, de Wit PJGM (2009) Fungal effector proteins. Annu Rev Phytopathol 47:233–263
Thines M, Lebeda A, Burdon JJ, Thrall P, Jege MJ (2014) Phylogeny and evolution of plant pathogenic oomycetes-a global overview. Eur J Plant Pathol 138:431–447
van Esse HP, Thomma BP, van 't Klooster JW, de Wit PJ, (2006) Affinity-tags are removed from Cladosporium fulvum effector proteins expressed in the tomato leaf apoplast. J Exp Bot 57:599–608
Wang Y, Tyler BM, Wang Y (2019) Defense and counterdefense during plant-pathogenic oomycete infection. Annu Rev Microbiol 73:30–31
Wawra S, Belmonte R, Löbach L, Saraiva M, Willems A, van West P (2012) Secretion, delivery and function of oomycete effector proteins. Curr Opin Microbiol 15:685–691
Zhang ZH, Huang SX, Tao H, Zhang Y, Zhao Y, Ji ZL, Chen XR (2019) Gene transcriptional pattern, prokaryotic expression and functional analysis of an apoplastic, hydrophobic and small effector SCR82 from Phytophthora capsici. Acta Microbiol Sinica 59:1586–1599
Zhu J (2012) Mammalian cell protein expression for biopharmaceutical production. Biotechnol Adv 30:1158–1170
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31671971, 31871907), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. SJCX19_0895), the Open Project of Joint International Research Laboratory of Agriculture and Agri-Product Safety of Ministry of Education of China (No. JRK20180012), the Yangzhou University 2016 Project for Excellent Young Key Teachers, and High-Level Talent Support Program of Yangzhou University.
Supporting information
Supplementary Table 1—Primers used in the study. Supplementary Fig. 1—The gene cloning strategy, DNA and protein sequences of SCR96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, SX., Zhang, ZH., Liu, W. et al. Expression of the small cysteine-rich protein SCR96 from Phytophthora cactorum in mammalian cells: phytotoxicity and exploitation of its polyclonal antibody. Biotechnol Lett 42, 125–133 (2020). https://doi.org/10.1007/s10529-019-02754-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02754-7