Abstract
Objective
To identify the key residues of Thermus thermophilus (T. thermophilus) RTCB in RNA ligation and DNA activation.
Results
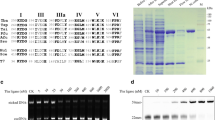
The biochemical activities of RTCB from T. thermophilus were purified, characterized, and compared. Structure and sequence alignment between T. thermophilus RTCB and Pyrococcus horikoshii (P. horikoshii) RTCB identified six conserved residues (D64, D95, N203, H204, E207, H399) that were essential for RNA ligation. Mutation analysis showed that the expression levels of mutants D95A, N203A, H204A, E207A and H399A were relatively low. Compared to wide-type RTCB, variant D64A protein had no RNA ligation and DNA activation activity. In addition, T. thermophilus RTCB showed acceptable catalytic activity of 3′-phosphate DNA activation at 37 °C.
Conclusion
D64 was proved to be essential for RTCB-catalyzed RNA ligation and DNA activation (from 37 to 70 °C) in T. thermophilus.




Similar content being viewed by others
References
Burroughs AM, Aravind L (2016) RNA damage in biological conflicts and the diversity of responding RNA repair systems. Nucleic Acids Res 44:8525–8555
Chakravarty AK, Shuman S (2012) The sequential 2’,3′-cyclic phosphodiesterase and 3′-phosphate/5′-OH ligation steps of the RtcB RNA splicing pathway are GTP-dependent. Nucleic Acids Res 40:8558–8567
Chakravarty AK, Subbotin R, Chait BT, Shuman S (2012) RNA ligase RtcB splices 3′-phosphate and 5′-OH ends via covalent RtcB-(histidinyl)-GMP and polynucleotide-(3′)pp(5′)G intermediates. Proc Natl Acad Sci USA 109:6072–6077
Das U, Chakravarty AK, Remus BS, Shuman S (2013) Rewriting the rules for end joining via enzymatic splicing of DNA 3′-PO4 and 5′-OH ends. Proc Natl Acad Sci USA 110:20437–20442
Desai KK, Cheng CL, Bingman CA, Phillips GN Jr, Raines RT (2014) A tRNA splicing operon: Archease endows RtcB with dual GTP/ATP cofactor specificity and accelerates RNA ligation. Nucleic Acids Res 42:3931–3942
Desai KK, Beltrame AL, Raines RT (2015) Coevolution of RtcB and Archease created a multiple-turnover RNA ligase. RNA 21:1866–1872
Englert M, Sheppard K, Aslanian A, Yates JR 3rd, Söll D (2011) Archaeal 3′-phosphate RNA splicing ligase characterization identifies the missing component in tRNA maturation. Proc Natl Acad Sci USA 108:1290–1295
Huang J, Huo YY, Ji R, Kuang S, Ji C, Xu XW, Li J (2016) Structural insights of a hormone sensitive lipase homologue Est22. Sci Rep 6:28550
Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, Ji C, Gan J, Xu XW, Li J (2017) Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci USA 114:10642–10647
Maughan WP, Shuman S (2015) Characterization of 3′-phosphate RNA ligase paralogs RtcB1, RtcB2, and RtcB3 from Myxococcus xanthus highlights DNA and RNA 5′-phosphate capping activity of RtcB3. J Bacteriol 197:3616–3624
Maughan WP, Shuman S (2016) Distinct contributions of enzymic functional groups to the 2′,3′-cyclic phosphodiesterase, 3′-phosphate guanylylation, and 3′-ppG/5′-OH ligation steps of the Escherichia coli RtcB nucleic acid splicing pathway. J Bacteriol 198:1294–1304
Nandy A, Saenz-Méndez P, Gorman AM, Samali A, Eriksson LA (2017) Homology model of the human tRNA splicing ligase RtcB. Proteins 85:1983–1993
Okada C, Maegawa Y, Yao M, Tanaka I (2006) Crystal structure of an RtcB homolog protein (PH1602-extein protein) from Pyrococcus horikoshii reveals a novel fold. Proteins 63:1119–1122
Pascal JM (2008) DNA and RNA ligases: structural variations and shared mechanisms. Curr Opin Struct Biol 18:96–105
Petree JR, Yehl K, Galior K, Glazier R, Deal B, Salaita K (2018) Site-selective RNA splicing nanozyme: DNAzyme and RtcB conjugates on a gold nanoparticle. ACS Chem Biol 13:215–224
Popow J, Englert M, Weitzer S, Schleiffer A, Mierzwa B, Mechtler K, Trowitzsch S, Will CL, Luhrmann R, Söll D, Martinez J (2011) HSPC117 is the essential subunit of a human tRNA splicing ligase complex. Science 331:760–764
Tanaka N, Shuman S (2011) RtcB is the RNA ligase component of an Escherichia coli RNA repair operon. J Biol Chem 286:7727–7731
Tanaka N, Meineke B, Shuman S (2011) RtcB, a novel RNA ligase, can catalyze tRNA splicing and HAC1 mRNA splicing in vivo. J Biol Chem 286:30253–30257
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFA0500600, 2015CB943300), the National Natural Science Foundation of China (31670878) and the Shanghai Committee of Science and Technology (18430711400) to J.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duan, S., Chen, Z., Li, Z. et al. Purification and enzymatic characterization of the RNA ligase RTCB from Thermus thermophilus. Biotechnol Lett 41, 1051–1057 (2019). https://doi.org/10.1007/s10529-019-02707-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02707-0