Abstract
Objective
To compare the effect of pre-propeptide (pre-pro) of the human prothrombin (hPT), with both the native and an R-9N mutant forms of the human factor IX (hFIX) pre-pro on the hFIX carboxylation, in Drosophila cell.
Results
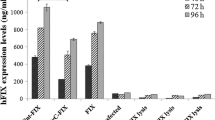
The three different pre-pro sequences, equipped with Drosophila Kozak, were joined to the mature hFIX cDNA and were subjected to transient expression analysis of hFIX in the S2 Drosophila cells, compared to that of a native hFIX cDNA, with its native Kozak. Replacement of the hFIX pre-pro sequence with that of hPT increased the biological activity of hFIX, significantly. The highest total level of hFIX expression occurred for the native hFIX with the Drosophila Kozak. However, the hFIX secretion efficiency with this construct was less than that of the native hFIX with its native Kozak. The R-9N substitution, in the hFIX propeptide, with no apparent effect on the FIX γ-carboxylation, reduced the FIX expression efficiency.
Conclusion
Potential of the hPT pre-pro sequence for FIX expression in Drosophila cells, was confronted by γ-glutamyl carboxylase (GGCX) saturation in ER, besides the functional importance of -9 amino acid in propeptide is described; these are noteworthy for production of γ-carboxylated proteins.





Similar content being viewed by others
References
Bandyopadhyay PK, Garrett JE, Shetty RP, Keate T, Walker CS, Olivera BM (2002) Gamma-glutamyl carboxylation: an extracellular posttranslational modification that antedates the divergence of molluscs, arthropods, and chordates. Proc Natl Acad Sci USA 99:1264–1269. https://doi.org/10.1073/pnas.022637099
Bandyopadhyay PK, Clark K, Stevenson BJ, Rivier JE, Olivera BM, Golic KG, Rong YS (2006) Biochemical characterization of Drosophila gamma-glutamyl carboxylase and its role in fly development. Insect Mol Biol 15:147–156. https://doi.org/10.1111/j.1365-2583.2006.00619.x
Berkner KL (2000) The vitamin K–dependent carboxylase. J Nutr 130:1877–1880
Blostein M, Cuerquis J, Landry S, Galipeau J (2008) The carboxylation efficiency of the vitamin K-dependent clotting factors: studies with factor IX. Haemophilia 14:1063–1068
Bunch TA, Grinblat Y, Goldstein LS (1988) Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res 16:1043–1061
Camire RM, Larson PJ, Stafford DW, High KA (2000) Enhanced gamma-carboxylation of recombinant factor X using a chimeric construct containing the prothrombin propeptide. Biochemistry 39:14322–14329
Cavener DR (1987) Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res 15:1353–1361
Galeffi P, Brownlee GG (1987) The propeptide region of clotting factor IX is a signal for a vitamin K dependent carboxylase: evidence from protein engineering of amino acid-4. Nucleic Acids Res 15:9505–9513
Haddad-Mashadrizeh A, Zomorodipour A, Izadpanah M, Sam MR, Ataei F, Sabouni F, Hosseini SJ (2009) A systematic study of the function of the human β-globin introns on the expression of the human coagulation factor IX in cultured Chinese hamster ovary cells The. J Gene Med 11:941–950
Johansen H, van der Straten A, Sweet R, Otto E, Maroni G, Rosenberg M (1989) Regulated expression at high copy number allows production of a growth-inhibitory oncogene product in Drosophila Schneider cells. Genes Dev 3:882–889
Jorgensen MJ, Cantor AB, Furie BC, Brown CL, Shoemaker CB, Furie B (1987) Recognition site directing vitamin K-dependent γ-carboxylation resides on the propeptide of factor IX. Cell 48:185–191
Kessler CM (2005) New perspectives in hemophilia treatment American Society of. Hematology 2005:429–435
Khorshidi S, Zomorodipour A, Behmanesh M, Vatandoost J, Bos MH (2015) Functional expression of the human coagulation factor IX using heterologous signal peptide and propeptide sequences in mammalian cell line. Biotechnol Lett 37:1773–1781
Kim YK, Shin HS, Tomiya N, Lee YC, Betenbaugh MJ, Cha HJ (2005) Production and N-glycan analysis of secreted human erythropoietin glycoprotein in stably transfected Drosophila S2 cells. Biotechnol Bioeng 92:452–461. https://doi.org/10.1002/bit.20605
Larson PJ, Stanfield-Oakley SA, VanDusen WJ, Kasper CK, Smith KJ, Monroe DM, High KA (1996) Structural integrity of the-carboxyglutamic acid domain of human blood coagulation Factor IXa is required for its binding to cofactor VIIIa. J Biol Chem 271:3869–3876
Li T, Yang CT, Jin D, Stafford DW (2000) Identification of a Drosophila vitamin K-dependent gamma-glutamyl carboxylase. J Biol Chem 275:18291–18296. https://doi.org/10.1074/jbc.M001790200
Lin PJ, Jin DY, Tie JK, Presnell SR, Straight DL, Stafford DW (2002) The putative vitamin K-dependent gamma-glutamyl carboxylase internal propeptide appears to be the propeptide binding site. J Biol Chem 277:28584–28591. https://doi.org/10.1074/jbc.M202292200
Liu J, Jonebring A, Hagström J, Nyström A-C, Lövgren A (2014) Improved expression of recombinant human factor IX by co-expression of GGCX, VKOR and Furin. Protein J 33:174–183
Mannucci PM, Tuddenham EG (2001) The hemophilias—from royal genes to gene therapy. N Engl J Med 344:1773–1779
Orlova N, Kovnir S, Vorobiev I, Gabibov A (2012) Coagulation factor IX for hemophilia B therapy. Acta Nat 4:13
Pan LC, Price PA (1985) The propeptide of rat bone gamma-carboxyglutamic acid protein shares homology with other vitamin K-dependent protein precursors. Proc Natl Acad Sci USA 82:6109–6113
Pipe SW (2008) Recombinant clotting factors. Thromb Haemost 99:840
Pudota BN, Miyagi M, Hallgren KW, West KA, Crabb JW, Misono KS, Berkner KL (2000) Identification of the vitamin K-dependent carboxylase active site: Cys-99 and Cys-450 are required for both epoxidation and carboxylation. Proc Natl Acad Sci USA 97:13033–13038
Reveillaud I, Niedzwiecki A, Bensch KG, Fleming JE (1991) Expression of bovine superoxide dismutase in Drosophila melanogaster augments resistance of oxidative stress. Mol Cell Biol 11:632–640
Schaub RG (2011) Recent advances in the development of coagulation factors and procoagulants for the treatment of hemophilia. Biochem Pharmacol 82:91–98
Schneider I (1972) Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27:353–365
Soejima Y et al (2013) Comparison of signal peptides for efficient protein secretion in the baculovirus-silkworm system. Open Life Sci 8:1–7
Stanley TB, Wu S-M, Houben RJ, Mutucumarana VP, Stafford DW (1998) Role of the propeptide and γ-glutamic acid domain of factor IX for in vitro carboxylation by the vitamin K-dependent carboxylase. Biochemistry 37:13262–13268
Stanley TB, Humphries J, High KA, Stafford DW (1999a) Amino acids responsible for reduced affinities of vitamin K-dependent propeptides for the carboxylase. Biochemistry 38:15681–15687
Stanley TB, Jin DY, Lin PJ, Stafford DW (1999b) The propeptides of the vitamin K-dependent proteins possess different affinities for the vitamin K-dependent carboxylase. J Biol Chem 274:16940–16944
Tie J-K, Zheng M-Y, Pope RM, Straight DL, Stafford DW (2006) Identification of the N-linked glycosylation sites of vitamin K-dependent carboxylase and effect of glycosylation on carboxylase function. Biochemistry 45:14755–14763
Vatandoost J, Zomorodipour A, Sadeghizadeh M, Aliyari R, Bos MH, Ataei F (2012) Expression of biologically active human clotting factor IX in Drosophila S2 cells: gamma-carboxylation of a human vitamin K-dependent protein by the insect enzyme. Biotechnol Prog 28:45–51. https://doi.org/10.1002/btpr.723
Wajih N, Hutson SM, Owen J, Wallin R (2005) Increased production of functional recombinant human clotting factor IX by baby hamster kidney cells engineered to overexpress VKORC1, the vitamin K 2,3-epoxide-reducing enzyme of the vitamin K cycle. J Biol Chem 280:31603–31607
Yao S-N, Wilson JM, Nabel EG, Kurachi S, Hachiya HL, Kurachi K (1991) Expression of human factor IX in rat capillary endothelial cells: toward somatic gene therapy for hemophilia B. Proc Natl Acad Sci USA 88:8101–8105
Zhang L, Leng Q, Mixson AJ (2005) Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J Gene Med 7:354–365
Acknowledgements
This study was supported by a grant (Project No. 372) from the National Institute of Genetic Engineering and Biotechnology of Iran.
Supporting information
Supplementary Table 1—List of the used oligonucleotides. Restriction sites are shown in gray. The Drosophila Kozak sequence is underlined.
Author information
Authors and Affiliations
Contributions
SB, AZ, and MGh: substantial contributions to the conception and design of the work. S.B, and AZ: contributions to the acquisition, analysis and interpretation of data for the study. SB: contributions to drafting the work. SB, AZ: revised the manuscript for important intellectual content. SB: conducted final approval of the version to the published. AZ: was accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bahrami, S., Ghaffari, M. & Zomorodipour, A. Production of recombinant human factor IX by propeptide modification in Drosophila S2 cell line. Biotechnol Lett 41, 347–355 (2019). https://doi.org/10.1007/s10529-019-02643-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02643-z