Abstract
Objective
To strengthen NADH regeneration in the biosynthesis of l-2-aminobutyric acid (l-ABA).
Results
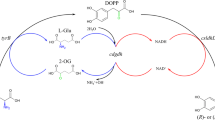
l-Threonine deaminase (l-TD) from Escherichia coli K12 was modified by directed evolution and rational design to improve its endurance to heat treatment. The half-life of mutant G323D/F510L/T344A at 42 °C increased from 10 to 210 min, a 20-fold increase compared to the wild-type l-TD, and the temperature at which the activity of the enzyme decreased by 50% in 15 min increased from 39 to 53 °C. The mutant together with thermostable l-leucine dehydrogenase from Bacillus sphaericus DSM730 and formate dehydrogenase from Candida boidinii constituted a one-pot system for l-ABA biosynthesis. Employing preheat treatment in the one-pot system, the biosynthesis of l-ABA and total turnover number of NAD+/NADH were 0.993 M and 16,469, in contrast to 0.635 M and 10,531 with wild-type l-TD, respectively.
Conclusions
By using the engineered l-TD during endured preheat treatment, the one-pot system has achieved a higher productivity of l-ABA and total turnover number of coenzyme.







Similar content being viewed by others
References
An Z, Gu X, Liu Y, Zhu Q (2017) Bioproduction of L-2-aminobutyric acid by a newly-isolated strain of Aspergillus tamarii ZJUT ZQ013. Catal Lett 147:837–844
Ansorge MB, Kula MR (1999) Production of recombinant l-leucine dehydrogenase from Bacillus cereus in pilot scale using the runaway replication system E. coli [pIET98]. Biotechnol Bioeng 65:557–562
Berríos-Rivera SJ, Bennett GN, San KY (2002) Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD+-dependent formate dehydrogenase. Metab Eng 4:217–229
Eisenstein E (1991) Cloning, expression, purification, and characterization of biosynthetic threonine deaminase from Escherichia coli. J Biol Chem 266:5801–5807
Galkin A, Kulakova L, Yoshimura T, Soda K, Esaki N (1997) Synthesis of optically active amino acids from α-keto acids with Escherichia coli cells expressing heterologous genes. Appl Environ Microb 63:4651–4656
Gallagher D, Gilliland G, Xiao G, Zondlo J, Fisher K, Chinchilla D, Eisenstein E (1998) Structure and control of pyridoxal phosphate dependent allosteric threonine deaminase. Structure 6:465–475
Hatfield GW, Umbarger HE (1970) Threonine deaminase from Bacillus subtilis. J Biol Chem 245:1742–1747
Heuser F, Schroer K, Lütz S, Bringer-Meyer S, Sahm H (2007) Enhancement of the NAD(P)(H) pool in Escherichia coli for biotransformation. Eng Life Sci 7:343–353
Krutsakorn B, Honda K, Ye X, Imagawa T, Bei X, Okano K, Ohtake H (2013) In vitro production of n-butanol from glucose. Metab Eng 20:84–91
Li Z, Tao R, Wang Y, Jiang Y, Lin X, Yang Y, Zheng H, Jiang W, Yang S (2011) Removal of l-alanine from the production of l-2-aminobutyric acid by introduction of alanine racemase and d-amino acid oxidase. Appl Microbiol Biotechnol 90:903–910
Park ES, Dong JY, Shin JS (2013) ω-Transaminase-catalyzed asymmetric synthesis of unnatural amino acids using isopropylamine as an amino donor. Org Biomol Chem 11:6929–6933
Pezeshgi Modarres H, Mofrad MR, Sanati-Nezhad A (2016) Protein thermostability engineering. RSC Adv 6:115252–115270
Qiao P, Wu M, Zhu L, Zhang Y, Yang L, Fei H, Lin J (2015) Enhancing the thermal tolerance of a cis-epoxysuccinate hydrolase via combining directed evolution with various semi-rational redesign methods. J Mol Catal B Enzym 121:96–103
Sin JS, Kim BG (2009) Transaminase-catalyzed asymmetric synthesis of l-2-aminobutyric acid from achiral reactants. Biotechnol Lett 31:1595–1599
Tao R, Jiang Y, Zhu F, Yang S (2014) A one-pot system for production of L-2-aminobutyric acid from l-threonine by l-threonine deaminase and a NADH-regeneration system based on l-leucine dehydrogenase and formate dehydrogenase. Biotechnol Lett 36:835–841
Yin L, Hu X, Xu D, Ning J, Chen J, Wang X (2012) Co-expression of feed-back-resistant threonine dehydratase and acetohydroxy acid synthase increase l-isoleucine production in Corynebacterium glutamicum. Metab Eng 14:542–550
Acknowledgements
This work was funded by the National Hi-Tech Research and Development Program of China (2014AA022105).
Supporting information
Supplementary Table 1—Bacterial strains and plasmids used in this study.
Supplementary Table 2—Primers used in this study.
Supplementary Table 3—Biotransformation of l-ABA by the original and revised systems.
Supplementary Fig. 1—Electronic interaction between substrate l-threonine and (a) wild-type l-TD or (b) G323D/F510L/T344A.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Li, GS., Qiao, P. et al. Increased productivity of l-2-aminobutyric acid and total turnover number of NAD+/NADH in a one-pot system through enhanced thermostability of l-threonine deaminase. Biotechnol Lett 40, 1551–1559 (2018). https://doi.org/10.1007/s10529-018-2607-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2607-3