Abstract
Objectives
To engineer Yarrowia lipolytica for improving the heterologous production of campesterol (a key precursor to manufacture pharmaceutical steroids).
Results
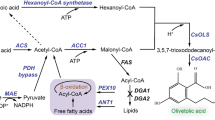
By screening 7-dehydrocholesterol reductase (DHCR7) from diverse species, DHCR7 from Danio rerio was the best candidate for campesterol synthesis. Overexpression of ACL (ATP: citrate lyase) or POX2 (peroxisome acyl-CoA oxidase 2) were key to improving campesterol production. The highest yield of campesterol was 942 mg/l was with the strain overexpressing POX2 in a 5 l bioreactor via high cell density fermentation process with a restricted supply of carbon sourc, sunflower seed oil.
Conclusions
A promising platform to synthesize downstream steroid drugs was established. Efficient approaches were provided to improve the production of desired molecules in Y. lipolytica with high oil utilization efficiency.



Similar content being viewed by others
References
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y (2016) Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact 15:113
Du HX, Xiao WH, Wang Y, Zhou X, Zhang Y, Liu D, Yuan YJ (2016) Engineering Yarrowia lipolytica for campesterol overproduction. PLoS ONE 11:e0146773
Duport C, Spagnoli R, Degryse E, Pompon D (1998) Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat Biotechnol 16:186–189
Friedlander J, Tsakraklides V, Kamineni A, Greenhagen EH et al (2016) Engineering of a high lipid producing Yarrowia lipolytica strain. Biotechnol Biofuels 9:77
Kocharin K, Chen Y, Siewers V, Nielsen J (2012) Engineering of acetyl-CoA metabolism for the improved production of polyhydroxybutyrate in Saccharomyces cerevisiae. AMB Express 2:1
Lecain E, Chenivesse X, Spagnoli R, Pompon D (1996) Cloning by metabolic interference in yeast and enzymatic characterization of Arabidopsis thaliana sterol D7-reductase. J Biol Chem 271:10866–10873
Luo YS, Nicaud JM, Van Veldhoven PP, Chardot T (2002) The acyl–CoA oxidases from the yeast Yarrowia lipolytica: characterization of Aox2p. Arch Biochem Biophys 403:32–38
Mlickova K, Roux E, Athenstaedt K, d’Andrea S, Daum G, Chardot T, Nicaud JM (2004) Lipid accumulation, lipid body formation, and acyl coenzyme A oxidases of the yeast Yarrowia lipolytica. Appl Environ Microbiol 70:3918–3924
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Papanikolaou S, Chevalot I, Komaitis M, Aggelis G, Marc I (2001) Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 80:215–224
Sestric R, Munch G, Cicek N, Sparling R, Levin DB (2014) Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient-sufficient and nutrient limited conditions. Bioresour Technol 164:41–46
Smith JJ, Brown TW, Eitzen GA, Rachubinski RA (2000) Regulation of peroxisome size and number by fatty acid beta-oxidation in the yeast Yarrowia lipolytica. J Biol Chem 275:20168–20178
Souza CM, Schwabe TM, Pichler H, Ploier B, Leitner E, Guan XL, Wenk MR, Riezman I, Riezman H (2011) A stable yeast strain efficiently producing cholesterol instead of ergosterol is functional for tryptophan uptake, but not weak organic acid resistance. Metab Eng 13:555–569
Wang G, Xiong X, Ghogare R, Wang P, Meng Y, Chen S (2016) Exploring fatty alcohol-producing capability of Yarrowia lipolytica. Biotechnol Biofuels 9:107
Yang X, Nambou K, Wei L, Hua Q (2016) Heterologous production of alpha-farnesene in metabolically engineered strains of Yarrowia lipolytica. Bioresour Technol 216:1040–1048
Yao K, Wang FQ, Zhang HC, Wei DZ (2013) Identification and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng 15:75–87
Zhou J, Yin X, Madzak C, Du G, Chen J (2012) Enhanced alpha-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J Biotechnol 161:257–264
Acknowledgements
This work was supported by the financial support from the International S&T Cooperation Program of China (2015DFA00960), the National Natural Science Foundation of China (21390203, 31570088 and 21622605) and Innovative Talents and Platform Program of Tianjin (16PTSYJC00050).
Supplementary information
Supplementary Table 1—Plasmids and strains used in this study.
Supplementary Table 2—Primers used in this study.
Supplementary Table 3—The Codon-optimized sequences of 7-dehydrocholesterol reductase (DHCR7) involved in this study.
Supplementary Fig. 1—Analysis of sterols profile of Y. lipolytica strains SyBE_Yl02060002 and SyBE_Yl02060004–08 for DHCR7 screening.
Supplementary Fig. 2—GC-TOF/MS analysis of campesterol producing strains SyBE_Yl02060006 and SyBE_Yl02060056.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Y., Yao, M. et al. Improved campesterol production in engineered Yarrowia lipolytica strains. Biotechnol Lett 39, 1033–1039 (2017). https://doi.org/10.1007/s10529-017-2331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2331-4