Abstract
Objectives
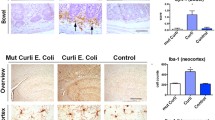
Involvement of the outer membrane protein C (OmpC) of Escherichia coli in neurodegeneration was investigated using a mouse model.
Results
OmpC formed protease-resistant fibres that exhibited the diagnostic features of an amyloid. The spectral shift in the Congo Red and the thioflavin T assays produced features similar to neurotoxic peptides. Intramuscular administration of OmpC in mice resulted in spongiform neurodegeneration of the brain through calcium-dependent apoptosis and also showed upregulation of apoptosis related genes. Immunolocalization of OmpC in brain demonstrated the direct involvement of the porin in neurodegeneration and formation of spongiform encephalopathy.
Conclusion
We have demonstrated the ability of OmpC of E. coli to induce neurodegeneration in mice.





Similar content being viewed by others
References
Basle A, Rummel G, Storici P, Rosenbusch JP, Schirmer T (2006) Crystal structure of osmoporin OmpC from E. coli at 2.0 A. J Mol Biol 362:933–942
Bieler S, Estrada L, Lagos R, Baeza M, Castilla J, Soto C (2005) Amyloid formation modulates the biological activity of a bacterial protein. J Biol Chem 280:26880–26885
Broxmeyer L (2004) Is mad cow disease caused by a bacteria? Med Hypoth 63:731–739
Buommino E, Morelli F, Metafora S, Rossano F, Perfetto B, Baroni A, Tufano MA (1999) Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect Immun 67:4794–4800
Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855
Del Puerto HL, Martins AS, Moro L, Milsted A, Alves F, Braz GF, Vasconcelos AC (2010) Caspase-3/-8/-9, Bax and Bcl-2 expression in the cerebellum, lymph nodes and leukocytes of dogs naturally infected with canine distemper virus. Genet Mol Res 9:151–161
Furukawa H, Doh-ura K, Okuwaki R, Shirabe S, Yamamoto K, Udono H, Ito T, Katamine S, Niwa M (2004) A pitfall in diagnosis of human prion diseases using detection of protease-resistant prion protein in urine. Contamination with bacterial outer membrane proteins. J Biol Chem 279:23661–23667
Glover JR, Kowal AS, Schirmer EC, Patino MM, Liu JJ, Lindquist S (1997) Self-seeded fibers formed by Sup 35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89:811–819
Hetz C, Bono MR, Barros LF, Lagos R (2002) Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci USA 99:2696–2701
Howie AJ, Brewer DB, Howell D, Jones AP (2008) Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab Invest 88:232–242
Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276:5960–5972
Kagan BL, Azimov R, Azimova R (2004) Amyloid peptide channels. J Membr Biol 202:1–10
Kim KS (2002) Strategy of Escherichia coli for crossing the blood-brain barrier. J Infect Dis 186:S220–S224
Koebnik R, Locher KP, Van Gelder P (2000) Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol Microbiol 37:239–253
Kourie JI, Culverson A (2000) Prion peptide fragment PrP[106-126] forms distinct cation channel types. J Neurosci Res 62:120–133
Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418:291
Lasmezas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J, Dormont D (1997) Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402–405
LeVine H 3rd (1993) Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 2:404–410
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1:120–129
Mittal R, Wang Y, Hunter CJ, Gonzalez-Gomez I, Prasadarao NV (2009) Brain damage in newborn rat model of meningitis by Enterobacter sakazakii: a role for outer membrane protein A. Lab Investig 89:263–277
Payne CM, Crowley C, Washo-Stultz D, Briehl M, Bernstein H, Bernstein C, Beard S, Holubec H, Warneke J (1998) The stress-response proteins poly(ADP-ribose) polymerase and NF-kappaB protect against bile salt-induced apoptosis. Cell Death Differ 5:623–636
Petersen A, Castilho RF, Hansson O, Wieloch T, Brundin P (2000) Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurons. Brain Res 857:20–29
Prusiner SB (1998) Prions. PNAS USA 95:13363–13383
Shaked GM, Shaked Y, Kariv-Inbal Z, Halimi M, Avraham I, Gabizon R (2001) A protease-resistant prion protein isoform is present in urine of animals and humans affected with prion diseases. J Biol Chem 276:31479–31482
Sigurdson CJ, Heikenwalder M, Manco G, Barthel M, Schwarz P, Stecher B, Krautler NJ, Hardt WD, Seifert B, MacPherson AJ, Corthesy I, Aguzzi A (2009) Bacterial colitis increases susceptibility to oral prion disease. J Infect Dis 199:243–252
Tamguney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, DeArmond SJ, Prusiner SB (2009) Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532
Taylor KL, Cheng N, Williams RW, Steven AC, Wickner RB (1999) Prion domain initiation of amyloid formation in vitro from native Ure2p. Science 283:1339–1343
Tufano MA, Berlingieri MT, Sommese L, Galdiero F (1984) Immune response in mice and effects on cells by outer membrane porins from Salmonella typhimurium. Microbiologica 7:353–366
Volles MJ, Lansbury PT Jr (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–7878
Wadsworth JD, Collinge J (2007) Update on human prion disease. Biochim et Biophy acta 1772:598–609
Wickner RB, Edskes HK, Ross ED, Pierce MM, Baxa U, Brachmann A, Shewmaker F (2004) Prion genetics: new rules for a new kind of gene. Ann Rev Genet 38:681–707
Xie Y, Kim KJ, Kim KS (2004) Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immun Med Microbiol 42:271–279
Acknowledgments
We thank the Directors of CIBA and ERI, for providing necessary facilities to carry out the experiments. We thank Dr. S Antony Ceasar, N. Kalaimani, S. V. Alavandi and M. Poornima for critical evaluation of the manuscript. Dr. V. Sankar, Dr. S. Skylab and Dr. Krubakaran for their histopathological analysis and D. L. Mohanlal for technical assistance. We are grateful to Prof. John Cullum for correcting the manuscript. We are indebted to Dr Shankar, NIMHANS, Bangalore for providing human brain tissues. We thank ERI for financial support.
Conflict of interest
The authors declare that there are no conflicts of interest.
Supporting information
Supplementary Table 1 Details of primers used for the qRT-PCR analysis.
Supplementary Fig. 1 Neuropathology of porin treated mice hippocampus and cerebellum.
Supplementary Fig. 2 Porin trafficking in the experimental mice.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joseph Sahaya Rajan, J., Chinnappan Santiago, T., Singaravel, R. et al. Outer membrane protein C (OmpC) of Escherichia coli induces neurodegeneration in mice by acting as an amyloid. Biotechnol Lett 38, 689–700 (2016). https://doi.org/10.1007/s10529-015-2025-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-2025-8