Abstract
Objectives
To engineer the yeast Saccharomyces cerevisiae for the heterologous production of linalool.
Results
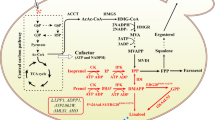
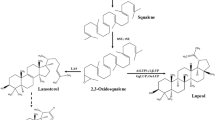
Expression of linalool synthase gene from Lavandula angustifolia enabled heterologous production of linalool in S. cerevisiae. Downregulation of ERG9 gene, that encodes squalene synthase, by replacing its native promoter with the repressible MET3 promoter in the presence of methionine resulted in accumulation of 78 µg linalool l−1 in the culture medium. This was more than twice that produced by the control strain. The highest linalool titer was obtained by combined repression of ERG9 and overexpression of tHMG1. The yeast strain harboring both modifications produced 95 μg linalool l−1.
Conclusions
Although overexpression of tHMG1 and downregulation of ERG9 enhanced linalool titers threefold in the engineered yeast strain, alleviating linalool toxicity is necessary for further improvement of linalool biosynthesis in yeast.

Similar content being viewed by others
References
Asadollahi MA, Maury J, Møller K, Nielsen KF, Schalk M, Clark A, Nielsen J (2008) Production of plant sesquiterpenes in Saccharomyces cerevisiae: effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol Bioeng 99:666–677
Asadollahi MA, Maury J, Patil KR, Schalk M, Clark A, Nielsen J (2009) Enhancing sesquiterpene production in Saccharomyces cerevisiae through in silico driven metabolic engineering. Metab Eng 11:328–334
Asadollahi MA, Maury J, Schalk M, Clark A, Nielsen J (2010) Enhancement of farnesyl diphosphate pool as direct precursor of sesquiterpenes through metabolic engineering of the mevalonate pathway in Saccharomyces cerevisiae. Biotechnol Bioeng 106:86–96
Behrendorff JBYH, Vickers CE, Chrysanthopoulos P, Nielsen LK (2013) 2,2-Diphenyl-1-picrylhydrazyl as a screening tool for recombinant monoterpene biosynthesis. Microb Cell Fact 12:76
Chen F, Tholl D, D’Auria JC, Farooq A, Pichersky E, Gershenzon J (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 15:481–494
Chen X, Yauk YK, Nieuwenhuizen NJ, Matich AJ, Wang MY, Perez RL, Atkinson RG, Beuning LL (2010) Characterisation of an (S)-linalool synthase from kiwifruit (Actinidia arguta) that catalyses the first committed step in the production of floral lilac compounds. Funct Plant Biol 37:232–243
Dudareva N, Cseke L, Blanc VM, Pichersky E (1996) Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8:1137–1148
Fischer MJC, Meyer S, Claudel P, Bergdoll M, Karst F (2011) Metabolic engineering of monoterpene synthesis in yeast. Biotechnol Bioeng 108:1883–1892
He X, Zhang B, Tan H (2003) Overexpression of a sterol C-24(28) reductase increases ergosterol production in Saccharomyces cerevisiae. Biotechnol Lett 25:773–778
Herrero Ó, Ramón D, Orejas M (2008) Engineering the Saccharomyces cerevisiae isoprenoid pathway for de novo production of aromatic monoterpenes in wine. Metab Eng 10:78–86
Jongedijk E, Cankar K, Ranzijn J, van der Krol S, Bouwmeester H, Beekwilder J (2015) Capturing of the monoterpene olefin limonene produced in Saccharomyces cerevisiae. Yeast 32:159–171
Landmann C, Fink B, Festner M, Dregus M, Engel KH, Schwab W (2007) Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch Biochem Biophys 465:417–429
Liu J, Zhang W, Du G, Chen J, Zhou J (2013) Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae. J Biotechnol 168:446–451
Maury J, Asadollahi MA, Møller K, Clark A, Nielsen J (2005) Microbial isoprenoid production: an example of green chemistry through metabolic engineering. Adv Biochem Eng Biotechnol 100:19–51
Oswald M, Fischer M, Dirninger N, Karst F (2007) Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res 7:413–421
Pardo E, Rico J, Gil JV, Orejas M (2015) De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb Cell Fact 14:136
Rico J, Pardo E, Orejas M (2010) Enhanced production of a plant monoterpene by overexpression of the 3-hydroxy-3-methylglutaryl coenzyme A reductase catalytic domain in Saccharomyces cerevisiae. Appl Environ Microbiol 76:6449–6454
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MCY, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Scalcinati G, Knuf C, Partow S, Chen Y, Maury J, Schalk M, Daviet L, Nielsen J, Siewers V (2012a) Dynamic control of gene expression in Saccharomyces cerevisiae engineered for the production of plant sesquitepene α-santalene in a fed-batch mode. Metab Eng 14:91–103
Scalcinati G, Partow S, Siewers V, Schalk M, Daviet L, Nielsen J (2012b) Combined metabolic engineering of precursor and co-factor supply to increase α-santalene production by Saccharomyces cerevisiae. Microb Cell Fact 11:117
Verduyn C, Postma E, Scheffers WA, van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517
Wang JF, Meng HL, Xiong ZQ, Zhang SL, Wang Y (2014) Identification of novel knockout and up-regulated targets for improving isoprenoid production in E. coli. Biotechnol Lett 36:1021–1027
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, Fickes S, Diola D, Benjamin KR, Keasling JD, Leavell MD, McPhee DJ, Renninger NS, Newman JD, Paddon CJ (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci USA 109:E111–E118
Yang J, Nie Q, Ren M, Feng H, Jiang X, Zheng Y, Liu M, Zhang H, Xian M (2013) Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene. Biotechnol Biofuels 6:60
Yuan JS, Köllner TG, Wiggins G, Grant J, Degenhardt J, Chen F (2008) Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J 55:491–503
Zhao L, Chang WC, Xiao Y, Liu HW, Liu P (2013) Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem 82:497–530
Zhou J, Wang C, Yoon SH, Jang HJ, Choi ES, Kim SW (2014) Engineering Escherichia coli for selective geraniol production with minimized endogenous dehydrogenation. J Biotechnol 169:42–50
Zhu B-Q, Cai J, Wang Z-Q, Xu X-Q, Duan C-Q, Pan Q-H (2014) Identification of a plastid-localized bifunctional nerolidol/linalool synthase in relation to linalool biosynthesis in young grape berries. Int J Mol Sci 15:21992–22010
Acknowledgments
This study was supported by a grant (No. 89/84468) from the Biotechnology Committee, University of Isfahan, Iran. The authors are grateful to Prof. Wilfried Schwab, Technical University of Munich, for the kind gift of plasmid bearing linalool synthase gene from Lavandula angustifolia. Dr. Hadi Parastar is also thanked for assistance with GC analysis.
Supporting Information
Supplementary Table 1—Primers used for the PCR amplifications.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amiri, P., Shahpiri, A., Asadollahi, M.A. et al. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol Lett 38, 503–508 (2016). https://doi.org/10.1007/s10529-015-2000-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-015-2000-4