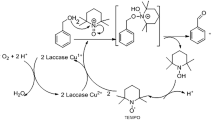
Abstract
Laccases play an important role in the biological break down of lignin and have great potential in the deconstruction of lignocellulosic feedstocks. We examined 16 laccases, both commercially prepared and crude extracts, for their ability to oxidize veratryl alcohol in the presence of various solvents and mediators. Screening revealed complete conversion of veratryl alcohol to veratraldehyde catalyzed by a crude preparation of the laccase from Trametes versicolor ATCC 11235 and the mediator TEMPO in 20 % (v/v) tert-butanol.

Similar content being viewed by others
References
Baiocco P, Barreca AN, Fabbrini M, Galli C, Gentili P (2003) Promoting laccase activity towards non-phenolic substrates: a mechanistic investigation with some laccase-mediator systems. Org Biomol Chem 1:191–197
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 2:215–242
Bourbonnais R, Paice MG (1990) Oxidation of nonphenolic substrates—an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Chakroun H, Mechichi T, Martinez MJ, Dhouib A, Sayadi S (2010) Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: application on bioremediation of phenolic compounds. Proc Biochem 43:507–513
d’Acunzo F, Galli C, Masci B (2002) Oxidation of phenols by laccase and laccase-mediator systems: solubility and steric issues. Eur J Biochem 269:5330–5335
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B Enzym 16:231–240
Fahraeus G, Reinhammar B (1967) Large-scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem Scand 21:2367–2378
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductase enzymes. Bioremediat J 3:1–26
Hilden K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31:1117–1128
Messerschmidt A, Huber R (1990) The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modeling and structural relationships. Eur J Biochem 2:341–352
Ride JP (1980) Physiol Plant Pathol 16:187–196
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 5:219–226
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable. USDA is an equal opportunity provider and employer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, T.M., Anderson, A.M. & Rich, J.O. Combinatorial evaluation of laccase-mediator system in the oxidation of veratryl alcohol. Biotechnol Lett 35, 225–231 (2013). https://doi.org/10.1007/s10529-012-1078-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1078-1