Abstract
Purpose of work:
The biosynthetic pathway of a new antitumor compound, haloroquinone, is elucidated to facilitate metabolic regulation for product accumulation and modification to produce new bioactive structural analogues of the compound.
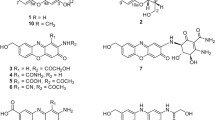
The biosynthetic origin of a novel promising protein kinase B inhibitor and anti-tumor compound, haloroquinone, from a marine-derived fungus, Halorosellinia sp. was clarified. The origin of carbon skeleton of haloroquinone was elucidated by feeding experiments with [2-13C]malonate and [1,2,3-13C3]malonate followed by 13C-NMR analysis of the isolated compounds: 15 carbon atoms were derived from malonate, of which eight were from the methylene group and seven from the carboxyl group. The remaining one is probably obtained by O-methylation. Haloroquinone is thus synthesized via a polyketide pathway using malonyl-CoA as both the starter unit and the extender unit.





Similar content being viewed by others
References
Barron PF, Bendall MR, Armstrong LG, Atkins AR (1984) Application of the DEPT pulse sequence for the generation of 13CHn subspectrum of coal-derived oils. Fuel 63:1276–1280
Bhadury P, Mohammad BT, Wright PC (2006) The current status of natural products from marine fungi and their potential as anti-infective agents. J Ind Microbiol Biotechnol 33:325–337
Fujii I, Mori Y, Watanabe A, Kubo Y, Tsuji G, Ebizuka Y (2000) Enzymatic synthesis of 1,3,6,8-tetradroxynaphthalene solely from malonyl-coenzyme A by a fungal iterative type I polyketide synthase PKS1. Biochemistry 39:8853–8858
Kang L, Cai M, Yu C, Zhang Y, Zhou X (2011) Improved production of the anticancer compound 1403C by glucose pulse feeding of marine Halorosellinia sp. (No. 1403) in submerged culture. Bioresour Technol 102:10750–10753
Kresze GB, Steber L, Oesterhelt D, Lynen F (1977) Reaction of yeast fatty acid synthetase with iodoacetamide. Eur J Biochem 79:173–180
Kubota T, Tsuda M, Kobayashi J (2001) Biosynthesis study of amphidinolide C. Tetrahedron 57:5975–5977
Ma SM, Li JWH, Choi JW, Zhou H, Lee KKM, Moorthie VA, Xie X, Kealey JT, Silva NAD, Vederas JC, Tang Y (2009) Complete reconstitution of a highly reducing iterative polyketide synthase. Science 326:589–592
McDaniel R, Welch M, Hutchinson CR (2005) Genetic approaches to polyketide antibiotic. Chem Rev 105:543–558
Rawlings BJ (1997) Biosynthesis of polyketide. Nat Prod Rep 14:523–556
Schneider B, Gershenzon J, Graser G, Hölscher D, Schmitt B (2003) One-dimensional 13C NMR and HPLC-1H NMR technique for observing carbon-13 and deuterium labeling in biosynthetic studies. Phytochem Rev 2:31–43
Shen B (2000) Biosynthesis of aromatic polyketides. Top Curr Chem 209:1–51
Shen B (2003) Polyketide biosynthesis beyond the type I, II and III polyketide synthase paradigms. Curr Opin Chem Biol 7:285–295
Thomas R (2001) A biosynthetic classification of fungal and streptomycete fused-ring aromatic polyketide. Chem Bio Chem 2:617–627
Walker TE, London RE, Whaley TW, Barker R, Matwiyoff NA (1976) Carbon-13 nuclear magnetic resonance spectroscopy of [1-13C] enriched monosaccharides. Signal assignments and orientation dependence of geminal and vicinal carbon–carbon and carbon–hydrogen spin–spin coupling constants. J Am Chem Soc 98:5807–5813
Xie G, Zhu X, Li Q, Gu M, He Z, Wu J, Li J, Lin Y, Li M, She Z, Yuan J (2010) SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br J Pharmacol 159:689–697
Yu C, Zhou X, Kang L, Zou W, Zhang Y (2010) Effects of pathway enzyme inhibitors and precursors on the anticancer compound 1403C synthesis in Halorosellinia sp. (No.1403). Biotechnol Bul 11:199–205
Yu C, Cai M, Kang L, Zhang Y, Zhou X (2012) Significance of seed culture methods on mycelial morphology and production of a novel anti-cancer anthraquinone by marine mangrove endophytic fungus Halorosellinia sp. (No. 1403). Proc Biochem 47:422–427
Acknowledgments
This work was financially supported by the National High Technology Research and Development Program of China (No. 2011AA090702; 2012AA092103; 2012AA092105) and Shanghai Leading Academic Discipline Project (Project Number: B505). We thank Prof. Zhigang She from Sun Yat-sen University for his friendly supply of Halorosellinia sp. (No. 1403).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, C., Cai, M., Zhang, Y. et al. Biosynthetic origin of the carbon skeleton of a novel anti-tumor compound, haloroquinone, from a marine-derived fungus, Halorosellinia sp.. Biotechnol Lett 34, 2119–2124 (2012). https://doi.org/10.1007/s10529-012-1019-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1019-z