Abstract
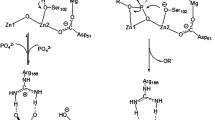
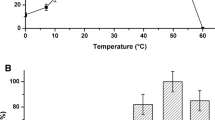
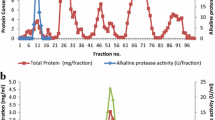
Treatment of a hyperthermophilic enzyme, alkaline phosphatase from Pyrococcus furiosus (PfuAP), with EDTA completely deactivated PfuAP, indicating that the presence of one or more divalent metal ions is essential for its catalytic activity. Subsequent addition of various divalent metal ions to the apoprotein recovered the enzymatic activity and, in particular, the addition of Co(II) resulted in an over 50-fold increase in activity compared with PfuAP before EDTA treatment. Intriguingly, PfuAP with Co(II) exhibited weaker stability toward heat treatment, suggesting that Co2+ destabilizes the tertiary structure of PfuAP at high temperature.



Similar content being viewed by others
References
Atomi H (2005) Recent progress towards the application of hyperthermophiles and their enzymes. Curr Opin Chem Biol 9:166–173
Ensinger HA, Pauly HE, Pfleiderer G, Stiefel T (1978) The role of Zn(II) in calf intestinal alkaline phosphatase studied by the influence of chelating agents and chemical modification of histidine residues. Biochim Biophys Acta 527:432–441
Kim EE, Wyckoff HW (1991) Reaction mechanism of alkaline phosphatase based on crystal structures two-metal ion catalysis. J Mol Biol 218:449–464
Kitaoka M, Tsuruda Y, Tanaka Y, Goto M, Mitsumori M, Hayashi K, Hiraishi Y, Miyawaki K, Noji S, Kamiya N (2011) Transglutaminase-mediated synthesis of a DNA-(enzyme) n probe for highly sensitive DNA detection. Chem Eur J 17:5387–5392
Lingwood D, Ballantyne JS (2006) Alkaline phosphatase-immunoglobulin conjugate binds to lipids in vitro, independent of antibody selectivity. J Immunol Methods 311:174–177
Minamihata K, Tokunaga M, Kamiya N, Kiyoyama S, Sakuraba H, Ohshima T, Goto M (2009) Development of a novel immobilization method for enzymes from hyperthermophiles. Biotechnol Lett 31:1037–1041
Rahman RNZA, Fujiwara S, Takagi M, Kanaya S, Imanaka T (1997) Effect of heat treatment on proper oligomeric structure formation of thermostable glutamate dehydrogenase from a hyperthermophilic archaeon. Biochem Biophys Res Commun 241:646–652
Reynolds JA, Schlesinger MJ (1969) Formation and properties of a tetrameric form of Escherichia coli alkaline phosphatase. Biochemistry 8:4278–4282
Wojciechowski CL, Cardia JP, Kantrowitz ER (2002) Alkaline phosphatase from the hyperthermophilic bacterium T. maritima requires cobalt for activity. Protein Sci 11:903–911
Zappa S, Rolland JL, Flament D, Gueguen Y, Boudrant J, Dietrich J (2001) Characterization of a highly thermostable alkaline phosphatase from the euryarchaeon pyrococcus abyssi. Appl Environ Microbiol 67:4504–4511
Zappa S, Boudrant J, Kantrowitz ER (2004) Pyrococcus abyssi alkaline phosphatase: the dimer is the active form. J Inorg Biochem 98:575–581
Acknowledgments
This work was supported in part by the Science and Technology Incubation Program in Advanced Regions from the Japan Science and Technology Agency (JST). K. Minamihata was supported by a Research Fellowship of the Japan Society for the Promotion of Science (JSPS) for young scientists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minamihata, K., Goto, M. & Kamiya, N. Activation of Pyrococcus furiosus alkaline phosphatase by divalent metal ions. Biotechnol Lett 34, 2055–2060 (2012). https://doi.org/10.1007/s10529-012-0998-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0998-0