Abstract
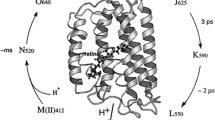
Bacteriorhodopsin (BR) mutagenesis plays an important role in the development of BR-based materials and tools with enhanced optical and electrical properties. Previously reported protocols for generating BR mutations are inefficient for the preparation and purification of mutant proteins. Therefore, a series of BR mutations were generated by using improved methods, which are described in further detail. The functional activity of the recombinant proteins was confirmed by spectroscopic and electrochemical assays. Modified proteins with different wavelengths and activities form a foundation for color-sensitive sensors and can be utilized to produce unique bioelectrical and biotechnological tools and materials. The proton-pumping activity of the generated mutant D85E was normal, indicating that the mutant could be used in light batteries. However, mutants D85Q and D85N were almost inactive; and D85N had a prolonged M state, suggesting that it could be utilized in light memories.






Similar content being viewed by others
References
Dunachtt M, Marti T, Khorana HG, Rothschild KJ (1990) UV-visible spectroscopy of bacteriorhodopsin mutants: substitution of Arg-82, Asp-85, Tyr-185, and Asp-212 results in abnormal light–dark adaptation. Biophysics 87:9873–9877
Giardi MT, Pace E (2005) Photosynthetic proteins for technological applications. Trends Biotechnol 23(5):257–263
Hampp N, Oesterhelt D (2004) Bacteriorhodopsin and its potential in technical applications. Nanbiotechnology 52:146–168
Hohenfeld IP, Wegener A, Engelhard M (1999) Purification of histidine tagged bacteriorhodopsin, pharaonis halorhodopsin and pharaonis sensory rhodopsin II functionally expressed in Escherichia coli. FEBS Lett 442(2–3):198–202 FEBS 21398
Kietis BP, Saudargas P, Vàro G, Valkunas L (2007) External electric control of the proton pumping in bacteriorhodopsin. Eur Biophys J 36:199–211
Korposh SO (2007) Development of sensitive elements based on photochromic bacteriorhodopsin for fiber optic sensors. Sens Actuators BioChem 133(1):281–290
Lanyi JK (2006) Proton transfers in the bacteriorhodopsin photocycle. Biochim Biophys Acta 1757:1012–1018
Lind Ch, Hojeberg B, Khorana HG (1981) Reconstitution of delipidated bacteriorhodopsin with endogenous polar lipid. J Biol Chem 256(16):8298–8305
Marti T (1998) Refolding of bacteriorhodopsin from expressed polypeptide fragments. J Biol Chem 273(15):9312–9322
Nabok A (2005) Organic and inorganic nanostructures. Artech House, Mass, pp 18–23
Peralvarez-Marin A (2005) Dynamic factors of bacteriorhodopsin: regulation key points in helices B, C and F. Thesis: University Autonoma Barcelona, Spain
Schobert B, Cupp-Vickery J, Hornak V, Smith SO, Lanyi JK (2002) Crystallographic structure of the K intermediate of bacteriorhodopsin. J Mol Biol 321:715–726
Subramaniam S, Marti T, Khorana HG (1990) Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82-Ala and Asp85-Glu. Biophysics 87:1013–1017
Tanio M, Inoue S, Yokota K, Seki T, Tuzi S, Needleman R, Lanyi JK, Naito A, Saito H (1999) Long-distance effects of site-directed mutations on backbone conformation in bacteriorhodopsin from solid state NMR of [1–13C]Val-labeled proteins. Biophys J 77:431–442
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saeedi, P., Moosaabadi, J.M., Sebtahmadi, S.S. et al. Site-directed mutagenesis in bacteriorhodopsin mutants and their characterization for bioelectrical and biotechnological equipment. Biotechnol Lett 34, 455–462 (2012). https://doi.org/10.1007/s10529-011-0731-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0731-4