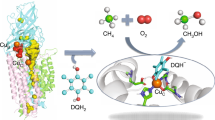
Abstract
The particulate methane monooxygenase (pMMO) of Methylosinus trichosporium OB3b oxidized n-butane and n-pentane and mainly produced (R)-2-butanol and (R)-2-pentanol that comprised 78 and 89% of the product, respectively, indicating that the pro-R hydrogen of the 2-position carbon of n-butane and n-pentane is oriented toward a catalytic site within the substrate binding site of pMMO. The protein cavity adjacent to the catalytic center for pMMO has optimum volume for recognizing n-butane and n-pentane for enantioselective hydroxylation.


Similar content being viewed by others
References
Bondi A (1964) Van der Waals volumes and radii. J Phys Chem 68:441–451
Burrows KJ, Cornish A, Scott D, Higgins IJ (1984) Substrate specificities of the soluble and particular methane monooxygenases of Methylosinus trichosporium OB3b. J Gen Microbiol 130:3327–3333
Chan SI, Wang VCC, Lai JCHL, Yu SSF, Chen PPY, Chen KHC, Chen CL, Chan MK (2007) Redox potentiometry studies of particulate methane monooxygenase: support for a trinuclear copper cluster active site. Angew Chem Int Ed 46:1992–1994
Corma A, Cavis M, Fornes V, Gonzalez-Alfaro V, Lobo R, Orchilles AV (1997) Cracking behavior of zeolites with connected 12- and 10-member ring channels: the influence of pore structure on product distribution. J Catal 167:438–446
Elliot SJ, Zhu M, Tso L, Nguyen HT, Yip JH, Chan SI (1997) Regio- and stereoselectivity of particulate methane monooxygenase from Methylococcus capsulatus (Bath). J Am Chem Soc 119:9949–9955
Funhoff EG, Van Beilen JB (2007) Alkane activation by P450 oxygenases. Biocatal Biotransform 25:186–194
Hakemian AS, Kondapalli KC, Telser J, Hoffman BM, Stemmler TL, Rosenzweig AC (2008) The metal centers of particulate methane monooxygenase from Methylosinus trichosporium OB3b. Biochemistry 47:6793–6801
Himes RA, Barnese K, Karlin KD (2010) One is lonely and three is a crowd: two coppers are for methane oxidation. Angew Chem Int Ed 49:6714–6716
Keener WK, Arp DJ (1993) Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol 59:2501–2510
Kerr JA (1966) Bond dissociation energy by kinetic methods. Chem Rev 66:465–500
Koshland D (1958) Application of a theory of enzyme specificity to protein synthesis. Proc Natl Acad Sci USA 44:98–104
Koyama T, Hayashi Y, Horie H, Kawauchi S, Matsumoto A, Iwase Y, Sakamoto Y, Miyaji A, Motokura K, Baba T (2010) Key role of the pore volume of zeolite for selective production of propylene from olefins. Phys Chem Chem Phys 12:2541–2554
Labinger JA (2004) Selective alkane oxidation: hot and cold approaches to a hot problem. J Mol Catal A Chem 220:27–35
Li Z, Chang D (2004) Recent advances in regio- and stereoselective hydroxylation of non-activated carbon atoms. Curr Org Chem 8:1647–1658
Lieberman LL, Rosenzweig AC (2005) Crystal structure of a membrane-bound metalloenzyme that catalyses the biological oxidation of methane. Nature 434:177–182
Liu R, Loll PJ, Eckenhoff RG (2005) Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle proteins. FASEB J 19:567–576
Martinho M, Choi DW, Dispirito AA, Antholine WE, Semrau JD, Munch E (2007) Mössbauer studies of the membrane-associated methane monooxygenase from Methylococcus capsulatus bath: evidence for a Diiron center. J Am Chem Soc 129:15783–15785
Mas-Ballesté R, Que L Jr (2006) Chemistry: targeting specific C–H bonds for oxidation. Science 312:1885–1886
Mecozzi S, Rebek J Jr (1998) The 55% solution: a formulation for molecular recognition in the liquid state. Chem Eur J 4:1016–1022
Miyaji A, Suzuki M, Baba T, Kamachi T, Okura I (2009) Hydrogen peroxide as an effecter on the inactivation of particulate methane monooxygenase under aerobic conditions. J Mol Catal B Enzym 57:211–215
Okazaki K, Takada S (2008) Dynamic energy landscape view of coupled binding and protein conformational change: induced-fit versus population-shift mechanisms. Proc Natl Acad Sci USA 105:11182–11187
Ono M, Okura I (1990) On the reaction mechanism of alkene epoxidation with Methylosinus trichosporium (OB3b). J Mol Catal 61:113–122
Park S, Shah NN, Taylor RT, Droege MW (1992) Batch cultivation of Methylosinus trichosporium OB3b: II. Production of particulate methane monooxygenase. Biotechnol Bioeng 40:151–157
Sugimori D, Ando R, Okura I (1995) Comparison of reactivity of alkane hydroxylation with intact cells and cell-free extracts of Methylosinus trichosporium OB3b. Appl Biochem Biotechnol 53:199–205
Yu SS, Wu LY, Chen KH, Luo WI, Huang DS, Chan SI (2003) The stereospecific hydroxylation of [2,2-2H2]butane and chiral dideuteriobutanes by the particulate methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem 278:40658–40669
Zürcher M, Gottschalk T, Meyer S, Bur D, Diederich F (2008) Exploring the flap pocket of the antimalarial target pasmepsin II: the “55% rule” applied to enzymes. ChemMedChem 3:237–240
Acknowledgment
This work was supported by a Grant-in-Aid for Young Scientists (B) (No. 20760527, 23760739), from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and partly by the Cooperative Research Program of Catalysis Research Center (CRC) Hokkaido University (Grant No. 10B2005). We thank Prof. Atsushi Fukuoka (CRC, Hokkaido University).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyaji, A., Miyoshi, T., Motokura, K. et al. The substrate binding cavity of particulate methane monooxygenase from Methylosinus trichosporium OB3b expresses high enantioselectivity for n-butane and n-pentane oxidation to 2-alcohol. Biotechnol Lett 33, 2241–2246 (2011). https://doi.org/10.1007/s10529-011-0688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0688-3