Abstract
Purpose of work
Soluble protein expression is an important first step during various types of protein studies. Here, we present the screening strategy of secretable mutant. The strategy aimed to identify those cysteine residues that provoke protein misfolding in the heterologous expression system.
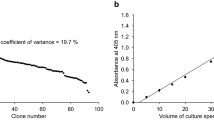
Intentional mutagenesis studies should consider the size of the library and the time required for expression screening. Here, we proposed a cysteine-to-serine shuffling mutation strategy (CS shuffling) using a Saccharomyces cerevisiae expression system. This strategy of site-directed shuffling mutagenesis of cysteine-to-serine residues aims to identify the cysteine residues that cause protein misfolding in heterologous expression. In the case of a nonglycosylated mutant of the taste-modifying protein miraculin (MCL), which was used here as a model protein, 25% of all constructs obtained from CS shuffling expressed MCL mutant, and serine mutations were found at Cys47 or Cys92, which are involved in the formation of the disulfide bond. This indicates that these residues had the potential to provoke protein misfolding via incorrect disulfide bonding. The CS shuffling can be performed using a small library and within one week, and is an effective screening strategy of soluble protein expression.


Similar content being viewed by others
References
Hagihara Y, Kim PS (2002) Toward development of a screen to identify randomly encoded, foldable sequences. Proc Natl Acad Sci USA 99:6619–6624
Ito K et al (2007) Microbial production of sensory-active miraculin. Biochem Biophys Res Commun 360:407–411
Ito K et al (2008) Advanced method for high-throughput expression of mutated eukaryotic membrane proteins in Saccharomyces cerevisiae. Biochem Biophys Res Commun 371:841–845
Kawamura S et al (2008) Role of disulfide bonds in goose-type lysozyme. FEBS J 275:2818–2830
Koschorreck K et al (2009) Improving the functional expression of a Bacillus licheniformis laccase by random and site-directed mutagenesis. BMC Biotechnol 9:12
Kurihara K, Beidler LM (1969) Mechanism of the action of taste-modifying protein. Nature 222:1176–1179
Liu Y et al (2005) Increase of soluble expression in Escherichia coli cytoplasm by a protein disulfide isomerase gene fusion system. Protein Expr Purif 44:155–161
Newstead S et al (2007) High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 104:13936–13941
Sakoh-Nakatogawa M et al (2009) Roles of protein-disulfide isomerase-mediated disulfide bond formation of yeast Mnl1p in endoplasmic reticulum-associated degradation. J Biol Chem 284:11815–11825
Sugawara T et al (2009) Fluorescence-based optimization of human bitter taste receptor expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun 382:704–710
Uchiyama H et al (2000) Directed evolution to improve the thermostability of prolyl endopeptidase. J Biochem 128:441–447
Yuan S et al (2004) The role of thioredoxin and disulfide isomerase in the expression of the snake venom thrombin-like enzyme calobin in Escherichia coli BL21 (DE3). Protein Expr Purif 38:51–60
Zamyatnin AA (1972) Protein volume in solution. Prog Biophys Mol Biol 24:107–123
Zavodszky M et al (2001) Disulfide bond effects on protein stability: designed variants of Cucurbita maxima trypsin inhibitor-V. Protein Sci 10:149–160
Acknowledgments
This study was supported in part by the ERATO Iwata Human Receptor Crystallography Project (JST) (to S.I.), the Targeted Proteins Research Program (to S.I. and T.M.), a Grant-in-Aid for Scientific Research (B) (20370035) (to T.K.), a Grant-in-Aid for Challenging Exploratory Research (22659059) (to T.K.), a grant from the Research and Development Program for New Bioindustry Initiatives (to K.A.), Grants-in-Aid for Scientific Research (S) (to K.A.), and Grants-in-Aid for JSPS Fellows (to K.I.) in Japan.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ito, K., Sugawara, T., Koizumi, A. et al. Cysteine-to-serine shuffling using a Saccharomyces cerevisiae expression system improves protein secretion: case of a nonglycosylated mutant of miraculin, a taste-modifying protein. Biotechnol Lett 33, 103–107 (2011). https://doi.org/10.1007/s10529-010-0399-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-010-0399-1