Abstract
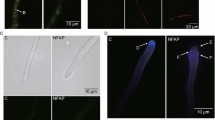
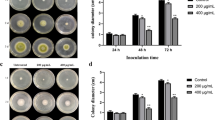
Antibacterial peptide, CM4 (ABP-CM4), a 35 amino acid peptide from Chinese silkworm—Bombyx mori, displayed a strong antifungal activity against Aspergillus niger, Trichoderma viride and Gibberella saubinetii. Scanning electron microcopy showed that the morphology of conidia became more irregular and swelled when treated with ABP-CM4 at its minimal inhibitory concentration (MIC) of 8 μM. A cell wall regeneration assay indicated that the plasma membrane was the prime target of ABP-CM4 action. Confocal laser scanning microscopy showed that the cytoskeleton of A. niger was destroyed when treated with ABP-CM4 at 8 μM. Furthermore, transmission electron microscopy showed that the membrane and the cellular organelles of fungus were disrupted and there were many vacuoles in the fungal cellular space after the treatment with ABP-CM4. A gel-retardation assay showed that ABP-CM4 bound the DNA of A. niger. Our results suggest that ABP-CM4 exerts its antifungal activity by disrupting the structure of cell membranes and the cytoskeleton and interacts with the organelles, such as the mitochondrion and with the DNA in the fungal cell, subsequently resulting in cell death.




Similar content being viewed by others
References
Christensen B, Fink J, Merrifield RB, Mauzerall D (1988) Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA 85:5072–5076
DeLucca AJ, Walsh TJ (1999) Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 43:1–11
Delucca AJ, Bland JM, Jacks TJ, Grimm C, Cleveland TE, Walsh TJ (1997) Fungicidal activity of cecropin A. Antimicrob Agents Chemother 41:481–483
Delucca AJ, Bland JM, Jacks TJ, Grimm C, Walsh TJ (1998) Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med Mycol 36:291–298
Diamond G (2001) Natures antibiotics: the potential of antimicrobial peptides as new drugs. Biologist (London) 48:209–212
Fang W, Shuangquan Z (1998) Studies on the action of the ABP-CM4 anti K562 cancer cells by SCGE. Prog Biochem Biophys 1:64–67
Hancock RE, Lehrer RI (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16:82–88
Huang HW (2000) Action of antimicrobial peptides: two-state model. Biochemistry 39:8347–8352
Jinshu X, Shuangquan Z (2001) The research of the mechanism of antibacterial peptide CM4 component against A. parasiticus. Prog Nat Sci 11:1263–1267
Karpova TS, Moltz SL, Riles LE, Gueldener U, Hegemann JH, Veronneau S, Bussey H, Cooper JA (1998) Depolarization of the actin cytoskeleton is a specific phenotype in Saccharomyces cerevisiae. J Cell Sci 111:2689–2696
Lee DG, Park Y, Kim HN, Kim HK, Kim PI, Choi BH, Hahm KS (2002) Antifungal mechanism of an antimicrobial peptide, HP (2-20), derived from N-terminus of Helicobacter pylori ribosomal protein L1 against Candida albicans. Biochem Biophys Res Commun 291:1006–1013
Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW (1996) Membrane pores induced by magainin. Biochemistry 35:13723–13728
Merrifield RB (1986) Solid phase synthesis. Science 232:341–347
Park Y, Hahm KS (2005) Effects of N- and C-terminal truncation of HP (2-20) from Helicobacter pylori ribosomal protein L1 (RPL1) on its anti-microbial activity. Biotechnol Lett 27:193–199
Park CB, Kim HS, Kim SC (1998) Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun 244:253–257
Shai Y, Oren Z (2001) From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides 22:1629–1641
Shuangquan Z, Hongwu J, Zhuyin D (1997) Ultrastructure observation of K562 leukemia cells treated with antibacterial peptide CM4 component. Prog Biochem Biophys 24:159–163
YiZeng T, Shuangquan Z, Xianming Q (1989) Separation, purification of antibacterial CM4 and the research of the structure and character. Sci China B 32:473–480
Zhong Z, Pirofski L (1998) Antifungal activity of a human anti-glucuronoxylomannan antibody. Clin Diagn Lab Immunol 5:58–64
Acknowledgements
This study was supported by Natural Science Foundation of JiangSu Province, China (No. BK2006221) and National Natural Sciences Foundation of China (30271093).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wu, X. & Zhang, SQ. Antifungal mechanism of antibacterial peptide, ABP-CM4, from Bombyx mori against Aspergillus niger . Biotechnol Lett 30, 2157–2163 (2008). https://doi.org/10.1007/s10529-008-9819-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9819-x