Abstract
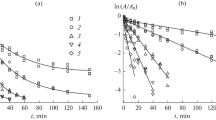
d-Amino acid oxidases from Rhodosporidium toruloides and Trigonopsis variabilis (RtDAO and TvDAO) are both yeast homodimeric flavoenzymes. Two of their cDNA genes were connected by a hexanucleotide linker and heterologously expressed in E. coli to produce the corresponding double DAOs (dRtDAO and dTvDAO) with two subunits fused into a single polypeptide. The specific activities of double DAOs remained similar to those of native dimeric DAOs, although the catalytic efficiencies (kcat/KM) were decreased due to higher KM values. The Tm value for dRtDAO was shifted 5°C higher while that for dTvDAO was increased only by 2°C, in comparison with the corresponding native counterparts. In the presence of 10 mM H2O2, dRtDAO and dTvDAO exhibited half-lives of about 60 and 40 min, respectively, which were 2- and 1.5-fold, respectively, longer than their native DAOs. These yeast DAOs can therefore be thermally and oxidatively stabilized by linking their subunits together.




Similar content being viewed by others
References
Curti B, Ronchi S, Pilone Simonetta M (1992) d- and l-Amino acid oxidases. In: Muller F (ed) Chemistry and biochemistry of flavoenzymes, vol 3. CRC Press, Boca Raton, pp 69–94
de la Mata I, Ramón F, Obregón V, Castillón MP, Acebal C (2000) Effect of hydrogen peroxide on d-amino acid oxidase from Rhodotorula gracilis. Enzyme Microb Technol 27:234–239
Fischer L (1998) d-Amino acid oxidases in biotechnology. Recent Res Dev Microbiol 2:295–317
Gabler M, Hensel M, Fischer L (2000) Detection and substrate selectivity of new microbial d-amino acid oxidase. Enzyme Microb Technol 27:605–611
Khang YH, Kim IW, Hah YR, Hwangbo JH, Kang KK (2003) Fusion protein of Viteroscilla hemoglobin with d-amino acid oxidase enhances activity and stability biocatalyst in the bioconversion process of cephalosporin C. Biotechnol Bioeng 82:480–488
Krebs HA (1935) Metabolism of amino-acids. III. Deamination of amino-acids. J Biochem 29:1620–1625
Liang H, Sandberg WS, Terwilliger TC (1993) Genetic fusion of subunits of a dimeric protein substantially enhances its stability and rate of folding. Proc Natl Acad Sci USA 90:7010–7014
Liao GJ, Lee YJ, Lee YJ, Chen LL, Chu WS (1998) Structure and expression of the d-amino acid oxidase gene from the yeast Rhodosporidiu toruloides. Biotechnol Appl Biochem 27:55–61
Nikolov A, Danielsson B (1994) Enzymatic transformation of cephalosporin-C to 7-amino-cephalosporanic acid. 2. Single-step procedure using a coimmobilized enzyme-system. Enzyme Microb Technol 16:1037–1041
Pilone MS (2000) d-Amino acid oxidase: new findings. Cell Mol Life Sci 57:1732–1747
Pilone MS, Pollegioni L (2002) d-Amino acid oxidase as an industrial biocatalyst. Biocatal Biotransformation 20:145–159
Pilone MS, Pollegioni L, Casalin P, Curti B, Ronchi S (1989) Properties of d-amino acid oxidase from Rhodotorula gracilis. Eur J Biochem 180:199–204
Pilone MS, Butò S, Pollegioni L (1995) A process for bioconversion of cephalosporin C by Rhodotorula gracilis d-amino acid oxidase. Biotechnol Lett 17:199–204
Piubelli L, Caldineli L, Molla G, Pilone MS, Pollegioni L (2002) Conversion of the dimeric d-amino acid oxidase from Rhodosporidium gracilis to a monomeric form. A rational mutagenesis approach. FEBS Lett 526:43–48
Piubelli L, Molla G, Caldinelli L, Pilone MS, Pollegioni L (2003) Dissection of the structural determinants involved in formation of the dimeric form of d-amino acid oxidase from Rhodotorula gracilis: role of the size of the βF5-βF6 loop. Protein Eng 16:1063–1069
Pollegioni L, Diederichs K, Molla G, Umhau S, Welte W, Ghisla S, Pilone MS (2002) Yeast d-amino acid oxidase: structural basis of its catalytic properties. J Mol Biol 324:535–546
Robinson CR, Sauer RT (1996) Covalent attachment of Arc repressor subunits by a peptide linker enhances affinity for operator DNA. Biochemistry 35:109–116
Roos V, Andersson CIJ, Arfvidsson C, Wahlund KG, Bülow L (2002) Expression of double Vitreoscilla hemoglobin enhances growth and alters ribosome and tRNA levels in Escherichia coli. Biotechnol Prog 18:652–656
Tishkov VI, Khoronenkova SV (2005) d-Amino acid oxidase: structure, catalytic mechanism, and practical application. Biochemistry 70:51–67
Umhau S, Pollegioni L, Molla G, Diederichs K, Welte W, Pilone MS, Ghisla S (2000) The X-ray structure of d-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent structure dehydrogenation. Proc Natl Acad Sci USA 97:12463–12468
Acknowledgments
We thank Professor Hao-Ping Chen in National Taipei University of Technology for providing d-amino acids. This research was supported by a grant NSC 95-2221-E-036-043 from the National Science Council of Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, SJ., Yu, CY., Lee, CK. et al. Subunit fusion of two yeast d-amino acid oxidases enhances their thermostability and resistance to H2O2 . Biotechnol Lett 30, 1415–1422 (2008). https://doi.org/10.1007/s10529-008-9694-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9694-5